TO THE EDITOR:
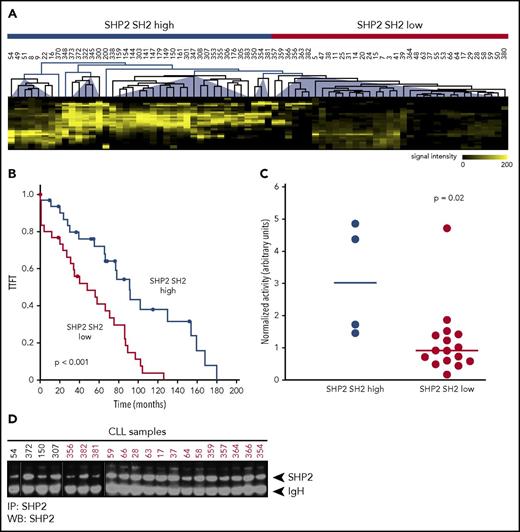
Anergy describes a state of B-cell receptor activation that leads to survival but not to proliferation in chronic lymphocytic leukemia (CLL) cells, and a high proportion of anergic cells correlates with an indolent clinical course. To gain deeper insights into anergic and proliferative CLL signaling, we performed Src homology 2 (SH2) domain profiling of phosphotyrosine-dependent signaling networks in a cohort of 34 untreated CLL patients by using the SH2 domains CRK, EAT2, GRB2, Pi3K, PLCγ, GAPN, SHIP2, and SHP2 (supplemental Figure 1A, available on the Blood Web site).1 This analysis revealed 4 signaling clusters that were clinically relevant, because patients grouped in clusters 1 to 3 (58.8%) showed significantly longer time to first treatment (TTFT) than those in cluster 4 (26.5%) (supplemental Figure 1B). A third group (14.7%) was only distantly related and showed high EAT2 SH2 binding activity. Of the applied SH2 domains, the phosphatase SHP2 mainly accounted for differences between clusters 1 to 3 and cluster 4 with more SHP2 binding sites correlating with longer TTFT as a surrogate marker for an indolent clinical course (clusters 1 to 3 have an SHP2 SH2 high signature; supplemental Figure 1B-C). This clinical correlation was reproducible in a second cohort of 40 CLL patients using only the SH2 domain of SHP2. Taken together, patients with an SHP2 SH2 high signature had a significantly longer TTFT in the overall cohort (Figure 1A-B), suggesting that signaling pathways involving SHP2 might contribute to CLL pathobiology and disease progression.
SH2 profiling of 74 primary CLL patients and clinical correlation. (A) Combined unsupervised cluster analysis of SH2 profiles of 74 primary CLL samples with the SHP2 SH2 domain. Far western blots (WBs) were scanned, images were digitalized, and lanes were horizontally subdivided into bins according to the molecular weight of the phosphoproteins. Hierarchical cluster analysis revealed 2 different clusters of low and high SHP2 SH2 binding. (B) TTFT Kaplan-Meier analysis according to SHP2 SH2 patient profiles (SHP2 SH2 high cluster: TTFT 96.2 ± 11.6 months; SHP2 SH2 low cluster: TTFT 50.9 ± 7.1 months; P < .001). (C) SHP2 activity determined after immunoprecipitation (IP) of SHP2 from 20 CLL samples of the high and low SHP2 SH2 clusters using 6,8 difluro-4-methylumbelliferyl phosphate as fluorogenic substrate. Fluorescence signals were normalized to SHP2 signals determined by immunoprecipitation and western blot analysis. Mean values of SHP2 activity are given as horizontal lines; level of significance was determined by the Wilcoxon rank-sum test. (D) SHP2 western blot analysis of the 20 CLL samples subsequent to SHP2 immunoprecipitation.
SH2 profiling of 74 primary CLL patients and clinical correlation. (A) Combined unsupervised cluster analysis of SH2 profiles of 74 primary CLL samples with the SHP2 SH2 domain. Far western blots (WBs) were scanned, images were digitalized, and lanes were horizontally subdivided into bins according to the molecular weight of the phosphoproteins. Hierarchical cluster analysis revealed 2 different clusters of low and high SHP2 SH2 binding. (B) TTFT Kaplan-Meier analysis according to SHP2 SH2 patient profiles (SHP2 SH2 high cluster: TTFT 96.2 ± 11.6 months; SHP2 SH2 low cluster: TTFT 50.9 ± 7.1 months; P < .001). (C) SHP2 activity determined after immunoprecipitation (IP) of SHP2 from 20 CLL samples of the high and low SHP2 SH2 clusters using 6,8 difluro-4-methylumbelliferyl phosphate as fluorogenic substrate. Fluorescence signals were normalized to SHP2 signals determined by immunoprecipitation and western blot analysis. Mean values of SHP2 activity are given as horizontal lines; level of significance was determined by the Wilcoxon rank-sum test. (D) SHP2 western blot analysis of the 20 CLL samples subsequent to SHP2 immunoprecipitation.
SHP2 is a phosphotyrosine phosphatase (PTP) encoded by the PTPN11 gene. Indeed, this PTP was expressed in virtually all CLL patients at varying levels of expression (supplemental Figure 2). However, SHP2 expression levels were not predictive for the SHP2 SH2 signature, suggesting that the activated phospho-tyrosyl bound forms rather than global protein levels (including the closed autoinhibitory conformation) were responsible for the clinical correlation. To substantiate this, we determined the phosphatase activity of SHP2 in primary CLL cells by using an assay based on 6,8 difluoro-4-methylumbelliferyl phosphate (supplemental Figure 3). Of note, only 4 of 20 available primary CLL samples showing the SHP2 SH2 high signature yielded sufficient SHP2 immunoprecipitate to be used in the phosphatase activity screen, but immunoprecipitations from 16 of 25 available samples showing the SHP2 SH2 low signature were successful. This result may be explained by significantly more competing binding sites for SHP2 in the SHP2 SH2 high cluster. In line with our hypothesis, the phosphatase activity screen of all available samples from the 2 clusters revealed a significantly increased phosphatase activity of SHP2 in CLL patients from the SHP2 SH2 high signature (Figure 1C-D).
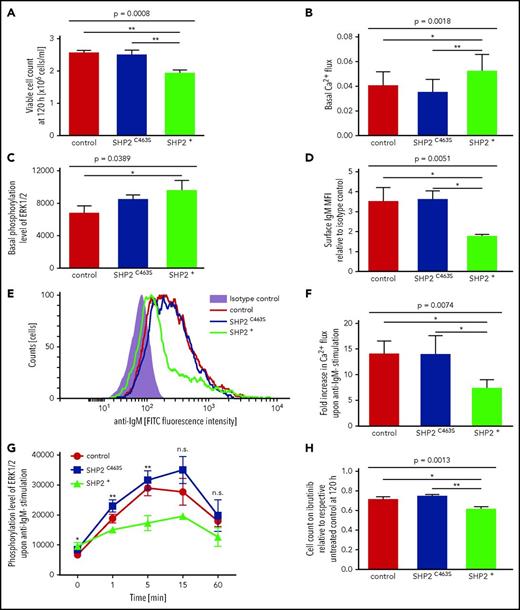
To further decipher the molecular mechanisms underlying the SHP2 signature, we used an MEC-1 knockdown and overexpression model as well as an OSU-CLL overexpression model, assuming that the knockdown would mimic the SHP2 SH2 low signature whereas overexpression of this protein would increase the SHP2 activity thereby mimicking, at least in part, the SHP2 SH2 high signature. In line with our hypothesis, the knockdown of SHP2 did not result in any proliferative effects, because SHP2 levels were already low in the parental MEC-1 cell line (supplemental Figure 4A-B). In contrast, overexpression of SHP2, but not a phosphatase-dead SHP2 mutant, resulted in reduced proliferation (Figure 2A). We used PamGene kinase profiling as an unbiased approach to pin down potential signaling pathways in which SHP2 could be involved in CLL. Kinase profiling pointed at Ca2+ and MAPK signaling as differentially activated in SHP2 overexpressing versus control MEC-1 cells (supplemental Figure 5). This prompted us to analyze SHP2 involvement in pathological BCR signaling, since an increase in intracellular Ca2+ concentration and enhanced MAPK signaling is one of the major initial steps in B-cell activation upon BCR engagement. Interestingly, overexpression of functional SHP2 resulted in a subtle increase of basal Ca2+ (Figure 2B) and MAPK signaling (Figure 2C) and in a downregulation of surface BCR (Figure 2D-E). Moreover, SHP2 overexpression mitigated responses to anti-immunoglobulin M (anti-IgM) stimulation such as Ca2+ mobilization as well as activation of downstream MAPK signaling (Figure 2F-G). Antiproliferative effects of ibrutinib were enhanced in MEC-1 cells overexpressing SHP2 (Figure 2H). Confirming the observations in the MEC-1 cell line model, reduced proliferation and Ca2+ mobilization were also observed in OSU-CLL cells overexpressing SHP2 in contrast to a phosphatase-dead mutant SHP2 (supplemental Figure 6A-B). However, these effects were smaller in comparison with the MEC-1 cell line model, which was presumably due to the low level of overexpression achieved in OSU-CLL and comparable, but very low, surface BCR expression between the different OSU-CLL sublines (supplemental Figure 6C-D).
Anergic signature induced by SHP2 overexpression. Hallmarks of anergy were detectable in a transduced MEC-1 cell line overexpressing wild-type SHP2 or dysfunctional C463S-mutant SHP2 in comparison with MEC-1 cells transduced with empty vector control. Groups were compared by analysis of variance using Bonferroni post hoc statistics. (A) Effect of SHP2 overexpression on proliferation. Viable cell count was measured via cell viability analyzer 120 hours after seeding (n = 9 per group). (B) Basal Ca2+ levels were measured via flow cytometry after FLUO-4 staining (n = 8 per group). (C) Basal ERK1/2 phosphorylation levels were analyzed by densitometry of western blots (n = 6 per group). (D-E) Surface IgM expression in comparison with isotype control analyzed via flow cytometry (n = 6 per group). (F) Ca2+ flux after FLUO-4 staining in response to stimulation with soluble anti-IgM-F(ab′)2 determined by flow cytometry analysis (n = 5 per group). (G) Stimulation with soluble anti-IgM-F(ab′)2–induced ERK1/2 phosphorylation was analyzed by densitometry of western blots. Cell lines were serum starved for 2 hours and stimulated for 0, 1, 5, 15, or 60 minutes. Corresponding time points were always analyzed on the same blots (n = 2 to 6 per group). (H) Antiproliferative effect of SHP2 overexpression in ibrutinib-treated cells. Cell lines were seeded in medium with and without ibrutinib (0.5 µM). Viable cell count was measured via cell viability analyzer and normalized to the respective untreated control cell counts at 120 hours (n = 9 per group). (A-D,F,H) Error bars show the standard error of the mean; *P < .05; **P < .001. FITC, fluorescein isothiocyanate.
Anergic signature induced by SHP2 overexpression. Hallmarks of anergy were detectable in a transduced MEC-1 cell line overexpressing wild-type SHP2 or dysfunctional C463S-mutant SHP2 in comparison with MEC-1 cells transduced with empty vector control. Groups were compared by analysis of variance using Bonferroni post hoc statistics. (A) Effect of SHP2 overexpression on proliferation. Viable cell count was measured via cell viability analyzer 120 hours after seeding (n = 9 per group). (B) Basal Ca2+ levels were measured via flow cytometry after FLUO-4 staining (n = 8 per group). (C) Basal ERK1/2 phosphorylation levels were analyzed by densitometry of western blots (n = 6 per group). (D-E) Surface IgM expression in comparison with isotype control analyzed via flow cytometry (n = 6 per group). (F) Ca2+ flux after FLUO-4 staining in response to stimulation with soluble anti-IgM-F(ab′)2 determined by flow cytometry analysis (n = 5 per group). (G) Stimulation with soluble anti-IgM-F(ab′)2–induced ERK1/2 phosphorylation was analyzed by densitometry of western blots. Cell lines were serum starved for 2 hours and stimulated for 0, 1, 5, 15, or 60 minutes. Corresponding time points were always analyzed on the same blots (n = 2 to 6 per group). (H) Antiproliferative effect of SHP2 overexpression in ibrutinib-treated cells. Cell lines were seeded in medium with and without ibrutinib (0.5 µM). Viable cell count was measured via cell viability analyzer and normalized to the respective untreated control cell counts at 120 hours (n = 9 per group). (A-D,F,H) Error bars show the standard error of the mean; *P < .05; **P < .001. FITC, fluorescein isothiocyanate.
The pattern of IgM downregulation with attenuated responses to BCR engagement along with enhanced basal MAPK signaling has been previously recognized as characteristic for anergic B cells.2,3 Anergy is one of the most important physiological mechanisms to silence autoreactive B cells.4 Whereas in normal B cells, this program leads to apoptosis in the long term,5 in CLL cells, it seems to result in lethargic survival without proliferation as a result of widespread overexpression of the anti-apoptotic protein BCL-2 in this disease.6,7 Conceptually, anergy may seem to be more desirable, and indeed a high proportion of anergic cells is correlated with an indolent clinical course in this disease.8 Yet anergy describes a functional rather than an irreversible state of the cell, and therefore anergic cells are potentially harmful if they ultimately re-enter the proliferative cycle.9,10 Such recirculating cells re-express high levels of surface IgM, show strong inducible anti-IgM responses, engage T-cell help, and ultimately proliferate in lymph node proliferation centers. The balance between positive signaling that leads to proliferation and negative signaling that leads to anergy is therefore of prognostic significance in CLL.9,11
Although the cellular hallmarks of anergy are well established, the molecular drivers of this signature have been insufficiently elucidated so far. Previous experiments showed that SHIP1 and SHP1 (2 downstream phosphatases of Lyn acting in parallel pathways) were found to be important for maintaining the anergic state in B cells.12 One underlying mechanism could be the dephosphorylation of PIP3 by SHIP1 and PTEN that antagonizes PI3K signaling.13 In line with this, a subset of anergic B cells seems to overexpress PTEN.14 The SHP1 phosphatase plays a role in anergic feedback inhibition downstream of the BCR, but recent evidence suggests that it plays a much more complex role in CLL as part of the signalosome that orchestrates survival signals.15 Although anergic CLL cells do show significantly enhanced ERK1/2 phosphorylation,16 previous work failed to identify the link between BCR pathway attenuation and MAPK signaling. The data presented here indicate that SHP2 may serve as a molecular regulator of anergy in CLL and may be the missing link between attenuated responses to BCR stimulation and enhanced basal MAPK signaling as schematically shown in supplemental Figure 7. Importantly, our data clearly demonstrate that these effects are mediated by the phosphatase domain of SHP2, because a phosphatase-dead mutant of SHP2 did not induce an anergic phenotype. Taken together, our data broaden our understanding of pathological BCR signaling in CLL and may be exploited for therapeutic targeting.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the FACS Core Facility of the University Medical Center Hamburg-Eppendorf for expert technical assistance.
This work was supported by grant BI 1711/4-1 from the Deutsche Forschungsgemeinschaft (M.B.) and by the Fördergemeinschaft Kinderkrebs-Zentrum Hamburg, Germany (S. Buhs, H.G., and P.N.). M.B. holds a professorship for immunological cancer research and therapy supported by the Hubertus Wald-Foundation, Hamburg, Germany.
Authorship
Contribution: M.B. designed the study, supervised the experiments, and wrote the manuscript; S.S. designed, performed, and interpreted the experiments and wrote the manuscript; S. Buhs and S. Bolz designed, performed, and interpreted experiments; H.G. performed experiments; L.v.W. analyzed data and wrote the manuscript; K.R. performed and interpreted experiments; B.F. critically revised the manuscript; and P.N. supervised the experiments, analyzed data, and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mascha Binder, Department of Oncology and Hematology, Bone Marrow Transplantation with Section Pneumology, Hubertus Wald Tumorzentrum–University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr 52, 22767 Hamburg, Germany; e-mail: m.binder@uke.de; and Peter Nollau, Research Institute Children’s Cancer Center and Department of Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Martinistr 52, Building N63, 2nd Floor, 20251 Hamburg, Germany; e-mail: nollau@uke.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal