Key Points
Canonical Notch signaling is dispensable for steady-state and posttransplantation myelopoiesis, as well as stress erythropoiesis.
Key lineage regulators and Notch target genes are expressed independent of canonical Notch signaling in myelo-erythropoiesis.
Abstract
Although an essential role for canonical Notch signaling in generation of hematopoietic stem cells in the embryo and in thymic T-cell development is well established, its role in adult bone marrow (BM) myelopoiesis remains unclear. Some studies, analyzing myeloid progenitors in adult mice with inhibited Notch signaling, implicated distinct roles of canonical Notch signaling in regulation of progenitors for the megakaryocyte, erythroid, and granulocyte-macrophage cell lineages. However, these studies might also have targeted other pathways. Therefore, we specifically deleted, in adult BM, the transcription factor recombination signal-binding protein J κ (Rbpj), through which canonical signaling from all Notch receptors converges. Notably, detailed progenitor staging established that canonical Notch signaling is fully dispensable for all investigated stages of megakaryocyte, erythroid, and myeloid progenitors in steady state unperturbed hematopoiesis, after competitive BM transplantation, and in stress-induced erythropoiesis. Moreover, expression of key regulators of these hematopoietic lineages and Notch target genes were unaffected by Rbpj deficiency in BM progenitor cells.
Introduction
Notch signaling is a highly conserved pathway important in multiple developmental processes, not the least in decisive lineage fate decisions.1-3 Canonical signaling through all Notch receptors (Notch 1-4) converges on the transcription factor recombination signal binding protein for immunoglobulin κ J region (Rbpj, also known as CSL).4 In the absence of the Notch intracellular domain, Rbpj binds to specific sites and recruits other co-repressors to form a repression complex that tightly controls Notch signaling output. The Rbpj-associated co-repressor complex is displaced by a co-activator complex upon binding of Notch intracellular domain to Rbpj,1 triggering activation of Notch target genes, such as members of the family of basic helix-loop-helix Hairy/enhancer of split-related proteins Hes1, Hes5, and Hey1, as well as Nrarp.4
The best established function of Notch signaling in hematopoiesis is its critical role in the emergence of definitive hematopoietic stem cells (HSCs) in the early embryo5 and in thymic T-cell development.6-8 Although Notch signaling has been suggested to enhance HSC regeneration after chemotherapy and transplantation into irradiated recipients,9 canonical Notch signaling has been shown to be dispensable for HSC homeostasis in the adult bone marrow (BM).10,11 More recent studies have, however, suggested more distinct roles of Notch signaling in regulation of adult steady-state BM hematopoiesis, specifically, megakaryocyte (Mk) and erythroid (E) progenitor cell development,12,13 as well as in suppressing granulocyte-macrophage (GM) progenitor expansion.12,14,15 Importantly, these findings have been extended to implicate dysregulated canonical Notch signaling in acute megakaryoblastic leukemia,16 as well as myeloid malignancies.14,15
Although these studies have implicated a key role of tightly regulated canonical Notch signaling in physiological Mk, E, and GM progenitor development and homeostasis in steady-state adult BM, and its dysregulation in myeloid and Mk leukemias, these findings were largely made through genetic approaches potentially also affecting regulatory pathways distinct from canonical Notch signaling.12-14 Herein, we therefore investigated in detail the phenotype of distinct stages of Mk, E, and GM progenitors in the BM of adult mice with a pan-hematopoietic deletion of Rbpj, essential for canonical signaling through all Notch receptors.2,7 Contrary to previous reports, our studies reveal that all investigated stages of Mk, E, and GM progenitors in adult BM develop and are replenished independent of canonical Notch signaling.
Methods
Mice
B6 CD45.1 mice were from Jackson Laboratories (Bar Harbor, ME). Rbpjfl/fl,7 Mx1-Cre,17 Vav-Cre,18 and Vwf-eGFP BAC19 mice have been described, and had all been backcrossed to a C57Bl/6 background. Adult Rbpjfl/flCretg/+ mice at an age range of 6 to 18 weeks were used in experiments as specified, with wild-type (WT) Rbpjfl/flCre+/+ and heterozygous Rbpjfl/+Cretg/+ controls, the latter to control for Cre expression, as Rbpj heterozygosity previously has been shown to have no effect on hematopoiesis.7,20 All mouse experiments were performed under United Kingdom Home Office authorization.
Poly(I:C) treatment
Rbpj Mx1-Cre mice were intraperitoneally injected with poly(I:C) (GE Healthcare, Little Chalfont, United Kingdom) every second day, for 6 days, at a dose of 200 μg per injection. Peripheral blood and bone marrow were collected 4 to 5 weeks after the last injection for assessment of cellularity, phenotype by flow cytometry, and gene expression, and for use in bone marrow transplantation experiments.
Phenylhydrazine treatment
Rbpj Vav-Cre mice were intraperitoneally injected with phenylhydrazine (PHZ; Sigma Aldrich, Gillingham, United Kingdom) at a concentration of 50 mg/kg on 2 consecutive days. Peripheral blood was collected before treatment (day 0) and 2 and 4 days after last injection and circulating blood cell numbers were assessed by Sysmex. Bone marrow and spleen were harvested 4 days after last PHZ injection to evaluate the E progenitor response by flow cytometry.
Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) analysis was performed on a LSR II analyzer, and cell sorting on a FACSAria IIu Special Order Research Products (BD Biosciences, San Jose, CA). For instrument details and information about specific antibodies, see supplemental Data, available on the Blood Web site.
Gene expression analysis
Gene expression analysis was performed by real-time reverse transcription-polymerase chain reaction on Fluidigm dynamic array (Biomark, Cambridge, United Kingdom), as described.21 For further details, see supplemental Data.
Progenitor colony assays
Quantification of circulating blood cells
Peripheral blood was collected from the tail vein of individual mice into EDTA-coated tubes. Platelet and red blood cell (RBC) counts per milliliter of blood were quantified with a Sysmex automated hematology analyzer.
Transplantation assay
Lethally irradiated (900 cGy, split dose) B6-SJL CD45 (CD45.1 or CD45.1/2) or Vwf-eGFP (CD45.1/2) mice were transplanted with 1 million BM Rbpj homozygously deficient or control test cells (CD45.2), along with 1 million wild-type (CD45.1 or Vwf-eGFP CD45.1/2) competitor BM cells, as previously described.23 Mice were considered reconstituted if they had a minimum of 0.1% test cell contribution relative to total nucleated cells and a minimum of 0.02% of each of the NK1.1−Mac1+ myeloid, NK1.1−Mac1−CD19+ B, and NK1.1−Mac1−CD4/CD8+ T-cell lineages.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software. The statistical significance of differences between samples was determined using the 2-tailed Mann-Whitney U test.
For quantitative gene expression analysis, transplantation experiments, in vitro colony assays, and counting of circulating blood cells mean values of 2 to 4 technical replicates per individual mouse and cell population were determined. The mean values of each mouse (biological replicates) were then used to calculate the mean and variation (standard error of the mean, or standard deviation) within each group and statistical significance.
Results
Maintenance of distinct stages of myelo-E progenitors occurs independent of canonical Notch signaling
To assess how canonical Notch signaling affects distinct adult stages of Mk, E, and GM progenitors, we took 2 approaches to specifically delete Rbpj, using the constitutive pan-hematopoietic Vav-Cre24 (Figure 1A-E,H-I) or the inducible Mx1-Cre (supplemental Figure 1A-E) as CRE drivers, and performed a well-established and detailed staging of GM, Mk, and E progenitors25 in the BM of adult mice with a loxP-flanked conditional allele for Rbpj.
BM cellularity was not affected in Rbpj-deficient mice (Figure 1A; supplemental Figure 1A), and in agreement with previous studies,10 Lineage-Sca-1+c-Kit+CD150+Flt3− HSCs were unaffected (Figure 1B; supplemental Figure 1B). A detailed FACS analysis of distinct stages of GM, Mk, and E progenitors25 revealed no distinct defects, at any progenitor stage of these lineages, in Rbpj-deficient mice (Figure 1C-D; supplemental Figure 1C). Of particular relevance for the proposed roles of Notch signaling in the Mk12 and E13 lineages, neither the pre-Mk-E nor the lineage restricted Mk-progenitors, pre-colony forming unit-E (PreCFU-Es), or CFU-Es were significantly affected (Figure 1C-D; supplemental Figure 1C). To demonstrate that this lack of a phenotype was not a result of HSCs or any myeloid progenitor stage escaping Rbpj deletion, we FACS purified HSCs and all GM, Mk, and E progenitor stages from Rbpj-deficient (Vav-Cre-induced and Mx1-Cre-induced) mice and verified a virtually complete deletion of Rbpj (>99% in all stem and progenitor cell populations) with both Cre approaches (Figures 1E; supplemental Figure 1D). In agreement with these phenotypic data, the number (Figures 1F-G) and size (data not shown) of GM, E, and Mk colonies generated from unfractionated BM cells, as well as circulating platelet and RBC counts (Figure 1H-I; supplemental Figure 1E), was also unaffected in Rbpj-deficient mice. As a functional control that Rbpj indeed was deleted, thymic progenitors, including the earliest thymic progenitors, were almost undetectable in Rbpj-deficient thymi (supplemental Figure 1F-G), in agreement with previous studies.7 Collectively, these findings support that canonical Notch signaling is not required for steady-state generation, maintenance, or stepwise differentiation of adult GM, Mk, and E progenitors.
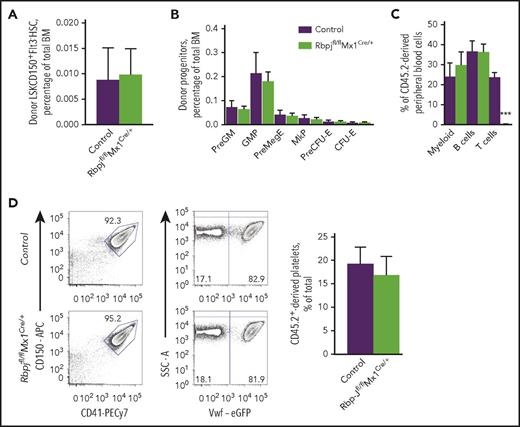
Unperturbed myelo-E progenitor hierarchies in steady-state BM of Rbpj-deficient mice. (A-D) Analysis of hematopoietic stem and myelo-E progenitor cells in 6- to 10-week-old Rbpjfl/flVavCre/+ (N = 8) and Rbpjfl/flVav+/+ (N = 4) and Rbpjfll+VavCre/+ (N = 2) littermate controls. (A) Mean (standard deviation [SD]) BM cellularity per 2 femurs and 2 tibias. (B-D) Representative FACS profiles and mean (SD) frequencies of (B) lineage−Sca-1+c-Kit+CD150+Flt3− HSCs and (C-D) myelo-E progenitor subsets. (E) Rbpj expression in HSCs and myelo-E progenitor subsets purified from 8-week-old Rbpjfl/flVavCre/+ (N = 5) and Rbpjfl/flVav+/+ or Rbpjfll+VavCre/+ littermate controls (N = 1 and 2, respectively). Mean (standard error of the mean [SEM]) expression normalized to hypoxanthine phosphoribosyltransferase 1 (Hprt). Samples in which the mean value of replicates was ≤0.001 (relative to Hprt) were considered below cutoff value (#). (F-G) In vitro colony assays using total BM from Rbpjfl/flMx1Cre/+ (N = 5-6) and age-matched (10-14-week-old) Rbpjfl/flMx1+/+ (N = 2) or Rbpjfl/+Mx1Cre/+ (N = 6) control mice treated with Poly(I:C) 4 to 5 weeks before analysis. (F) Mean (SD) in vitro CFU-GM and burst-forming units-E (BFU-E) colonies. (G) Pure (CFU-Mk) and mixed-lineage (Mk-mix) Mk-containing colonies scored after staining with acetylthiocholiniodide. Mean (SD) values from 2 to 3 experiments. (H-I) Mean (SEM [H] or SD [I]) of circulating platelet and red blood cell (RBC) counts in 6- to 10-week-old Rbpjfl/flVavCre/+ (N = 18-12) and age-matched controls (Rbpjfl/flVav+/+ [N = 8-11] or Rbpjfl/+VavCre/+ [N = 2-5]). For all data sets (A-I), statistical significance was investigated between homozygously Rbpj-deleted and control mice. *P < .05; **P < .01; ***P < .001. MegE, Mk-E progenitor; MkP, Mk progenitor; PreGM, pregranulocyte/macrophage progenitor; ProEry, pro-erythroblasts. See also supplemental Figure 1A-G.
Unperturbed myelo-E progenitor hierarchies in steady-state BM of Rbpj-deficient mice. (A-D) Analysis of hematopoietic stem and myelo-E progenitor cells in 6- to 10-week-old Rbpjfl/flVavCre/+ (N = 8) and Rbpjfl/flVav+/+ (N = 4) and Rbpjfll+VavCre/+ (N = 2) littermate controls. (A) Mean (standard deviation [SD]) BM cellularity per 2 femurs and 2 tibias. (B-D) Representative FACS profiles and mean (SD) frequencies of (B) lineage−Sca-1+c-Kit+CD150+Flt3− HSCs and (C-D) myelo-E progenitor subsets. (E) Rbpj expression in HSCs and myelo-E progenitor subsets purified from 8-week-old Rbpjfl/flVavCre/+ (N = 5) and Rbpjfl/flVav+/+ or Rbpjfll+VavCre/+ littermate controls (N = 1 and 2, respectively). Mean (standard error of the mean [SEM]) expression normalized to hypoxanthine phosphoribosyltransferase 1 (Hprt). Samples in which the mean value of replicates was ≤0.001 (relative to Hprt) were considered below cutoff value (#). (F-G) In vitro colony assays using total BM from Rbpjfl/flMx1Cre/+ (N = 5-6) and age-matched (10-14-week-old) Rbpjfl/flMx1+/+ (N = 2) or Rbpjfl/+Mx1Cre/+ (N = 6) control mice treated with Poly(I:C) 4 to 5 weeks before analysis. (F) Mean (SD) in vitro CFU-GM and burst-forming units-E (BFU-E) colonies. (G) Pure (CFU-Mk) and mixed-lineage (Mk-mix) Mk-containing colonies scored after staining with acetylthiocholiniodide. Mean (SD) values from 2 to 3 experiments. (H-I) Mean (SEM [H] or SD [I]) of circulating platelet and red blood cell (RBC) counts in 6- to 10-week-old Rbpjfl/flVavCre/+ (N = 18-12) and age-matched controls (Rbpjfl/flVav+/+ [N = 8-11] or Rbpjfl/+VavCre/+ [N = 2-5]). For all data sets (A-I), statistical significance was investigated between homozygously Rbpj-deleted and control mice. *P < .05; **P < .01; ***P < .001. MegE, Mk-E progenitor; MkP, Mk progenitor; PreGM, pregranulocyte/macrophage progenitor; ProEry, pro-erythroblasts. See also supplemental Figure 1A-G.
We next sought to address whether we could uncover a role for the canonical Notch pathway in regulation of GM, Mk, and E progenitors by establishing BM chimeras in which Rbpj-deficient progenitors compete with WT progenitors for replenishment and differentiation in lethally irradiated recipients. In this setting, BM stem and progenitor cells show increased cycling and turnover for months after transplantation.26,27 Notably, also in this competitive and regenerative setting, no deficiencies were observed in the replenishment of HSCs (Figure 2A), nor any stage of GM, Mk, and E progenitors in recipient mice transplanted with Rbpj-deficient as compared with WT BM cells (Figure 2B). Rbpj-deficient progenitors contributed equally well to replenishment of myeloid cells (Figure 2C), as well as platelets in peripheral blood, as WT control progenitors (Figure 2D). As expected, no T cells were found in the blood of Rbpj-deficient mice (Figure 2C).
Canonical Notch signaling is dispensable for stem and myelo-E progenitor cell replenishment in competitive bone marrow chimeras. (A-C) One million BM cells were harvested from 10- to 14-week-old Rbpjfl/flMx1Cre/+ and control (Rbpjfl/flMx1+/+ and Rbpjfl/+Mx1Cre/+) mice (CD45.2) 4 to 5 weeks after poly(I:C) treatment and transplanted into lethally irradiated wild-type (WT; CD45.1 or CD45.1/2) recipients together with 1 million competitor WT (CD45.1 or CD45.1/2) adult BM cells. Reconstitution of HSC (N = 2 of Rbpjfl/flMx1Cre/+ and N = 1 and N = 3 of Rbpjfl/flMx1+/+ and Rbpjfl/+Mx1Cre/+, respectively) and myeloid progenitor subsets (N = 5 of Rbpjfl/flMx1Cre/+, N = 2 of Rbpjfl/flMx1+/+, N = 3 of Rbpjfl/+Mx1Cre/+) in the BM of engrafted mice was assessed 7 to 9 weeks after transplantation. Percentage donor (CD45.2)-derived reconstitution of (A) HSC (mean ± SD) and (B) myeloid progenitor subsets (mean ±SEM) relative to total BM cells. (C) Mean (SEM) CD45.2 contribution of Rbpjfl/flMx1Cre/+ (N = 6) and control (N = 2 and N = 6 of Rbpjfl/flMx1+/+ and Rbpjfl/+Mx1Cre/+, respectively) BM cells to total NK1.1−Mac1+ myeloid, NK1.1−Mac1−CD19+ B and NK1.1−Mac1−CD4/CD8+ T cells. (D) Reconstitution of CD45.2-derived blood platelets (CD41+CD150+eGFP−) of total platelets in Vwf-eGFP (CD45.1/2) recipients 4 to 5 weeks after transplantation with 1 million 10- to 11-week-old CD45.2 Rbpjfl/flMx1Cre/+ (N = 4) or control Rbpjfl/+Mx1Cre/+ (N = 6) BM cells and 1 million Vwf-eGFP (CD45.1/2) competitor BM cells. (Left) Representative FACS profiles of platelet reconstitution in engrafted mice. (Right) Mean (SEM) percentage of CD150+CD41+ platelets derived from transplanted CD45.2 BM cells. For all data sets (A-D), statistical significance was investigated between Rbpj-deleted and control mice. ***P < .001.
Canonical Notch signaling is dispensable for stem and myelo-E progenitor cell replenishment in competitive bone marrow chimeras. (A-C) One million BM cells were harvested from 10- to 14-week-old Rbpjfl/flMx1Cre/+ and control (Rbpjfl/flMx1+/+ and Rbpjfl/+Mx1Cre/+) mice (CD45.2) 4 to 5 weeks after poly(I:C) treatment and transplanted into lethally irradiated wild-type (WT; CD45.1 or CD45.1/2) recipients together with 1 million competitor WT (CD45.1 or CD45.1/2) adult BM cells. Reconstitution of HSC (N = 2 of Rbpjfl/flMx1Cre/+ and N = 1 and N = 3 of Rbpjfl/flMx1+/+ and Rbpjfl/+Mx1Cre/+, respectively) and myeloid progenitor subsets (N = 5 of Rbpjfl/flMx1Cre/+, N = 2 of Rbpjfl/flMx1+/+, N = 3 of Rbpjfl/+Mx1Cre/+) in the BM of engrafted mice was assessed 7 to 9 weeks after transplantation. Percentage donor (CD45.2)-derived reconstitution of (A) HSC (mean ± SD) and (B) myeloid progenitor subsets (mean ±SEM) relative to total BM cells. (C) Mean (SEM) CD45.2 contribution of Rbpjfl/flMx1Cre/+ (N = 6) and control (N = 2 and N = 6 of Rbpjfl/flMx1+/+ and Rbpjfl/+Mx1Cre/+, respectively) BM cells to total NK1.1−Mac1+ myeloid, NK1.1−Mac1−CD19+ B and NK1.1−Mac1−CD4/CD8+ T cells. (D) Reconstitution of CD45.2-derived blood platelets (CD41+CD150+eGFP−) of total platelets in Vwf-eGFP (CD45.1/2) recipients 4 to 5 weeks after transplantation with 1 million 10- to 11-week-old CD45.2 Rbpjfl/flMx1Cre/+ (N = 4) or control Rbpjfl/+Mx1Cre/+ (N = 6) BM cells and 1 million Vwf-eGFP (CD45.1/2) competitor BM cells. (Left) Representative FACS profiles of platelet reconstitution in engrafted mice. (Right) Mean (SEM) percentage of CD150+CD41+ platelets derived from transplanted CD45.2 BM cells. For all data sets (A-D), statistical significance was investigated between Rbpj-deleted and control mice. ***P < .001.
Rbpj deficiency does not affect key Mk and E lineage programs nor Notch-related gene expression in Mk-E progenitor cells
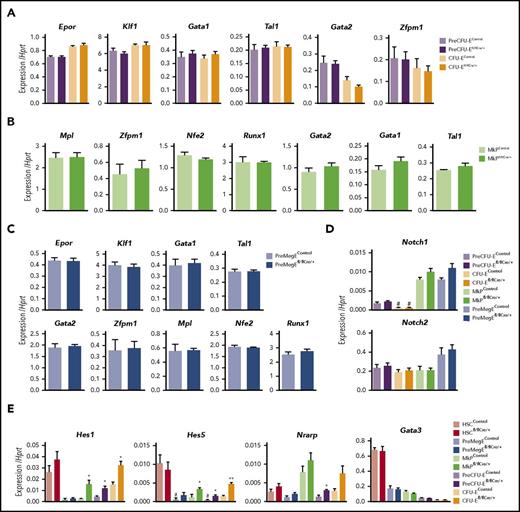
Although canonical Notch signaling did not appear to play any role for development or replenishment of any myelo-E progenitor subsets (Figures 1 and 2; supplemental Figure 1), we next investigated whether ablation of Rbpj might nevertheless affect expression of genes for key regulators of megakaryopoiesis and erythropoiesis at distinct progenitor stages for these lineages, as previously implicated.12,13 Notably, quantitative gene expression analysis demonstrated that transcript levels of a number of genes encoding critical Mk and E regulators were unaffected in Rbpj-deficient Mk and E progenitors (Figure 3A-C). Expression of Notch1 and Notch2 was also unaffected in Rbpj-deficient Mk and E progenitors (Figure 3D), and Notch3 and Notch4 transcripts were not detected in either WT nor Rbpj-deficient Mk and E progenitors (data not shown). As expression of Notch downstream target genes in myeloid, Mk, and E differentiation has been suggested to reflect activation through Notch signaling,12,13 we next, in contrast to previous studies, investigated whether the expression of key Notch target genes (Hes1, Hes5, Nrarp, and Gata3) in Mk and E progenitors in adult BM, was in fact dependent on, or potentially expressed independent of, canonical Notch signaling (Figure 3E; supplemental Figure 1H). Neither in HSCs nor in any Mk and E progenitor cells was the typically low expression of these Notch genes negatively affected by Rbpj deficiency. On the contrary, Hes1 and Hes5 were upregulated in the absence of Rbpj in Mk progenitors, pre-CFU-Es, and CFU-Es, suggesting Rbpj may serve as a transcriptional repressor of these genes in adult Mk and E progenitors in the “Notch off” state.
Expression of myelo-E lineage programs and Notch-target genes in Rbpj-deficient E and Mk progenitors. (A-D) E and Mk progenitor subsets were purified from individual 10- to 12-week-old Rbpjfl/flMx1Cre/+ (N = 6) and age-matched Rbpjfl/flMx1+/+ (N = 2) and Rbpjfl/+Mx1Cre/+ (N = 2) poly(I:C)-treated control mice and subjected to quantitative gene expression analysis for (A,C) erythroid and (B-C) megakaryocytic-related genes and (D) Notch1 and Notch2 receptors. Mean (SEM) values normalized to Hprt. No differences between Rbpjfl/flMx1Cre/+ and control mice reached statistical significance. (E) BM HSCs, Mk, and E progenitor cells (100 cells per replicate) were purified from individual 8-week-old RbpJfl/flVavCre/+ (N = 5) and age-matched RbpJfl/flVav+/+ or RbpJfl/+VavCre/+ controls (N = 1 and 2, respectively) and analyzed for expression of the Notch target genes Hes1, Hes5, Nrarp, and Gata3. Mean (SEM) values. Samples in which the mean value of replicates was ≤0.001 (relative to Hprt expression) were considered below cutoff value (#). For all data sets (A-E), statistical significance was investigated between Rbpj-deleted and control mice. *P < .05; **P < .01. See also supplemental Figure 1H.
Expression of myelo-E lineage programs and Notch-target genes in Rbpj-deficient E and Mk progenitors. (A-D) E and Mk progenitor subsets were purified from individual 10- to 12-week-old Rbpjfl/flMx1Cre/+ (N = 6) and age-matched Rbpjfl/flMx1+/+ (N = 2) and Rbpjfl/+Mx1Cre/+ (N = 2) poly(I:C)-treated control mice and subjected to quantitative gene expression analysis for (A,C) erythroid and (B-C) megakaryocytic-related genes and (D) Notch1 and Notch2 receptors. Mean (SEM) values normalized to Hprt. No differences between Rbpjfl/flMx1Cre/+ and control mice reached statistical significance. (E) BM HSCs, Mk, and E progenitor cells (100 cells per replicate) were purified from individual 8-week-old RbpJfl/flVavCre/+ (N = 5) and age-matched RbpJfl/flVav+/+ or RbpJfl/+VavCre/+ controls (N = 1 and 2, respectively) and analyzed for expression of the Notch target genes Hes1, Hes5, Nrarp, and Gata3. Mean (SEM) values. Samples in which the mean value of replicates was ≤0.001 (relative to Hprt expression) were considered below cutoff value (#). For all data sets (A-E), statistical significance was investigated between Rbpj-deleted and control mice. *P < .05; **P < .01. See also supplemental Figure 1H.
Canonical Notch signaling is dispensable for stress erythropoiesis
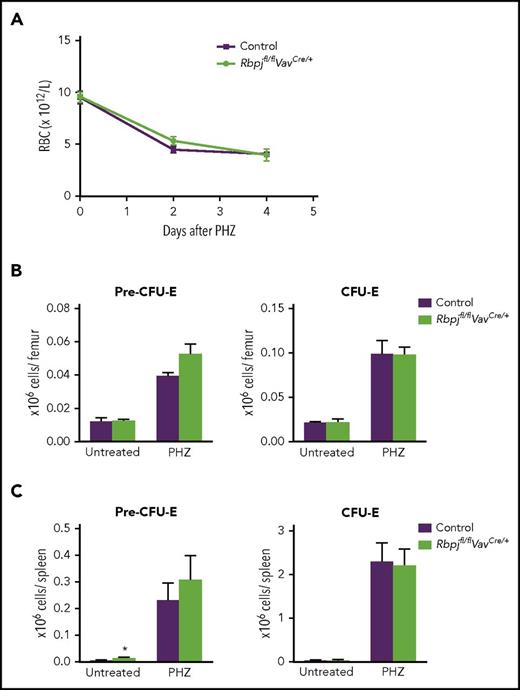
Notch signaling has been suggested to be critical for stress erythropoiesis.13 Because Rbpj deficiency showed no influence on replenishment of E progenitors after transplantation, we next assessed the effect of Rbpj deficiency on mature circulating RBCs as well as the reactive regeneration of E progenitors after PHZ-induced hemolytic anemia. The reduction in RBC numbers was not aggravated by Rbpj deficiency (Figure 4A), and E progenitors devoid of Rbpj were not affected in their ability to rapidly expand in response to PHZ-induced anemia, neither in BM nor spleen (Figure 4B-C). Therefore, reactive erythropoiesis in response to PHZ-induced anemia occurs independent of canonical Notch signaling.
Canonical Notch signaling is dispensable for phenylhydrazine-induced stress erythropoiesis. Seven- to 8-week-old RbpJfl/flVavCre/+ (N = 3-6) and age-matched RbpJfl/flVav+/+ or RbpJfl/+VavCre/+ controls (N = 2-3 and 2-3, respectively) were injected with PHZ to induce acute hemolytic anemia. (A) Circulating red blood cell counts in steady-state (day 0) and 2 and 4 days after last PHZ injection. Mean (SD) values are shown. (B-C) Mean (SEM) absolute numbers of E progenitors (PreCFU-E and CFU-E), 4 days after PHZ-induced anemia in BM (B) and spleen (C). *P < .05.
Canonical Notch signaling is dispensable for phenylhydrazine-induced stress erythropoiesis. Seven- to 8-week-old RbpJfl/flVavCre/+ (N = 3-6) and age-matched RbpJfl/flVav+/+ or RbpJfl/+VavCre/+ controls (N = 2-3 and 2-3, respectively) were injected with PHZ to induce acute hemolytic anemia. (A) Circulating red blood cell counts in steady-state (day 0) and 2 and 4 days after last PHZ injection. Mean (SD) values are shown. (B-C) Mean (SEM) absolute numbers of E progenitors (PreCFU-E and CFU-E), 4 days after PHZ-induced anemia in BM (B) and spleen (C). *P < .05.
Discussion
Several studies have implicated important and distinct roles of canonical Notch signaling in regulation of adult steady-state BM myelopoiesis, including megakaryopoiesis, erythropoiesis and the GM lineage,12,13 as well as its dysregulation in hematological malignancies of the megakaryocytic16 and GM14,15 lineages. Here, to more specifically address the role of canonical Notch signaling in GM, Mk, and E lineage development, we assessed the effect of deleting Rbpj, which encodes a DNA-binding protein through which canonical signaling from all 4 Notch receptors is relayed.
Our detailed mapping of distinct progenitor stages of the Mk, E, and GM lineages25 in Rbpj-deficient adult BM failed to support any role of canonical Notch signaling at any progenitor stage of each of these lineages. Although in disagreement with more recent studies,12-14 our findings are in line with earlier studies,7 which did not reveal any overt effect on myelopoiesis. Importantly, our findings were confirmed using 2 different Cre lines, both resulting in very efficient deletion of Rbpj, and phenocopying the characteristic early thymocyte block of deficient canonical Notch signaling.7,8 Rbpj deficiency also failed to affect the expression of investigated critical transcription factors and cytokine receptors in each of the relevant progenitor stages. Moreover, although we, in agreement with previous studies, confirmed expression of established Notch target genes in each of the investigated Mk, E, and GM progenitors, these were expressed at low levels and not downregulated on Rbpj deletion, indicating that canonical Notch signaling might not be active in these lineage-restricted progenitor stages.
We also investigated the effect of Rbpj deletion on replenishment of Mk, E, and GM progenitors after competitive BM transplantation of lethally irradiated recipients. Assessing the effect of Rbpj deficiency in BM chimeras has the advantage of potentially uncovering more subtle phenotypes by directly comparing the competiveness of Rbpj-deficient and WT progenitors. Despite of this, no BM Mk, E, or GM progenitor phenotype was revealed in transplanted Rbpj-deficient BM cells, nor was replenishment of mature myeloid cells or platelets in the blood affected by Rbpj deficiency. Because the turnover of HSCs and replenishment of hematopoietic progenitors occurs at enhanced speed after transplantation of BM-ablated recipients,26,27 our findings also suggest that canonical Notch signaling has little or no critical role in this regenerative setting. Moreover, in contrast to Oh et al,13 the robust reactive erythropoiesis seen in response to PHZ-induced anemia occurred independent of canonical Notch signaling. Nevertheless, although myelo-erythropoiesis in these regenerative settings was not affected by Rbpj deficiency, we cannot formally rule out that canonical signaling might be important for the myelo-E response to other challenges.
The explanation for the discrepancy between our findings and those based on alternative genetic approaches to inhibit Notch signaling12-14 remains unclear. Targeting of canonical Notch signaling, through transgenic expression of dnMAML1 in the studies implicating a role of canonical Notch signaling in megakaryopoiesis,12 may also result in simultaneous disruption of signaling pathways distinct from canonical Notch signaling. Likewise, although Nicastrin, a central component of the Notch signaling pathway, was deleted in the studies proposing a role of canonical Notch signaling in erythropoiesis and myelopoiesis,13,14 it also targets numerous other substrates, including CD44 and ErbB4.28-30 This suggests that some of the reported E and myeloid phenotypes in Nicastrin-deficient mice13,14 could also be attributed to altered processing of other target proteins, distinct from the Notch pathway. In that regard, it is plausible that our studies, through specifically deleting Rbpj, unlike these previous studies, have simply more specifically addressed the role of canonical Notch signaling. As such, our finding of a lack of a role of canonical Notch signaling in regulation of adult myeloid, E, and Mk lineage replenishment through a detailed investigation of the progenitor hierarchies of these lineages, in steady state as well as in perturbed adult hematopoiesis, is in agreement with early reports of unaffected myeloid colony-forming cells in Rbpj-deficient mice.7 The E and myeloid phenotypes reported after the specific and combined deletion of Notch1 and Notch2 or Notch1, Notch2, and Notch313,14 are, however, unlikely to be related to Notch-independent effects, but may instead be linked to noncanonical Notch signaling, although the E defect in Nicastrin-deficient mice was associated with reduced expression of implicated canonical Notch target genes.13,14 There is an increasing number of examples, also in the immune system, suggesting that Notch receptors can exert noncanonical functions independent of Rbpj.31-33 Regardless, it will be important to in further detail explore how abrogating Notch signaling at the receptor, but not at the canonical Rbpj level, results in the distinct Mk, E, and GM progenitor defects reported in previous studies.12-14
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Honjo for Rbp-Jfl/fl mice, the Biomedical Services at Oxford University for animal support, P. Sopp in the Weatherall Institute of Molecular Medicine (WIMM) FACS Facility (supported by the Medical Research Council (MRC) Human Immunology Unit, MRC Molecular Haematology Unit [MC_UU_12009], National Institute for Health Research Oxford Biomedical Research Center and the John Fell Fund [131/030 and 101/517], the Edward Penley Abraham Research Fund [CF182 and CF170], and the WIMM Strategic Alliance awards [G0902418 and MC_UU_12025]).
This work was supported by grants to S.E.W.J. from EU-FP7 StemExpand, EUFPEuroSyStem Integrated Project, the Medical Research Council UK (G0801073 and MC_UU_12009/5), and an international recruitment grant from the Swedish Research Council (2013-8995). S.E.W.J. and U.L. were supported by the Wallenberg Institute for Regenerative Medicine (supported by the Knut and Alice Wallenberg Foundation), and U.L. is supported by a project grant from the Swedish Research Council (K2014-64X-20097-09-5). S.D. was funded by Fundação para a Ciência e a Tecnologia (SFRH/BD/36816/2007). A.J.M. was supported by a Leukaemia and Lymphoma Research Senior Bennett Fellowship.
Authorship
Contribution: S.D. and S.E.W.J. conceptualized the study, with input from P.S.W., N.B.-V., C.N., and U.L.; S.D., P.S.W., and N.B.-V. designed the study methodology, with input from S.E.W.J.; data analysis and interpretation was conducted by S.D., P.S.W., N.B.-V., D.W.L.C., and S.E.W.J., with input from C.N. and U.L.; research was performed by S.D., P.S.W., N.B.-V., H.B., T.C.L., L.S., T.B.-J., H.F., A.J.M., D.A., S.J., S.-A.C., D.W.L.C., B.W., E.R., N.G., S.T., A.P.M., and Y.L.T., with input from S.E.W.J.; S.D., P.S.W., N.B.-V., and S.E.W.J. wrote the manuscript; S.E.W.J. supervised and acquired funding support for this work; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten Eirik W. Jacobsen, Hematopoietic Stem Cell Laboratory, Center for Hematology and Regenerative Medicine, Department of Medicine Huddinge, Karolinska Institutet, SE-141 86 Stockholm, Sweden; e-mail: sten.eirik.jacobsen@ki.se or sten.jacobsen@imm.ox.ac.uk.
References
Author notes
P.S.W. and N.B.-V. contributed equally to this article.

![Figure 1. Unperturbed myelo-E progenitor hierarchies in steady-state BM of Rbpj-deficient mice. (A-D) Analysis of hematopoietic stem and myelo-E progenitor cells in 6- to 10-week-old Rbpjfl/fl VavCre/+ (N = 8) and Rbpjfl/fl Vav+/+ (N = 4) and Rbpjfll+ VavCre/+ (N = 2) littermate controls. (A) Mean (standard deviation [SD]) BM cellularity per 2 femurs and 2 tibias. (B-D) Representative FACS profiles and mean (SD) frequencies of (B) lineage−Sca-1+c-Kit+CD150+Flt3− HSCs and (C-D) myelo-E progenitor subsets. (E) Rbpj expression in HSCs and myelo-E progenitor subsets purified from 8-week-old Rbpjfl/fl VavCre/+ (N = 5) and Rbpjfl/fl Vav+/+ or Rbpjfll+ VavCre/+ littermate controls (N = 1 and 2, respectively). Mean (standard error of the mean [SEM]) expression normalized to hypoxanthine phosphoribosyltransferase 1 (Hprt). Samples in which the mean value of replicates was ≤0.001 (relative to Hprt) were considered below cutoff value (#). (F-G) In vitro colony assays using total BM from Rbpjfl/fl Mx1Cre/+ (N = 5-6) and age-matched (10-14-week-old) Rbpjfl/fl Mx1+/+ (N = 2) or Rbpjfl/+ Mx1Cre/+ (N = 6) control mice treated with Poly(I:C) 4 to 5 weeks before analysis. (F) Mean (SD) in vitro CFU-GM and burst-forming units-E (BFU-E) colonies. (G) Pure (CFU-Mk) and mixed-lineage (Mk-mix) Mk-containing colonies scored after staining with acetylthiocholiniodide. Mean (SD) values from 2 to 3 experiments. (H-I) Mean (SEM [H] or SD [I]) of circulating platelet and red blood cell (RBC) counts in 6- to 10-week-old Rbpjfl/fl VavCre/+ (N = 18-12) and age-matched controls (Rbpjfl/fl Vav+/+ [N = 8-11] or Rbpjfl/+ VavCre/+ [N = 2-5]). For all data sets (A-I), statistical significance was investigated between homozygously Rbpj-deleted and control mice. *P < .05; **P < .01; ***P < .001. MegE, Mk-E progenitor; MkP, Mk progenitor; PreGM, pregranulocyte/macrophage progenitor; ProEry, pro-erythroblasts. See also supplemental Figure 1A-G.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-06-788505/4/m_blood788505f1.jpeg?Expires=1769174027&Signature=FzELW3~3isyG4mmP8im5KvNWJLjQ0OsD8B628h8UUS87tDc7YL6BvrEkX464aC~wl5LJERSNzZlhNFA8JVyjxOodOS7-dMcfF56Wn05v1Df0IUYDetGq1xVMPLS8aPWoFeuSqwc1ZHfWpEsquYyUtg3yTBnfLkeqrmJNyIUZlyYcorD5IlOdj3O34C0aM1wJ~S1~zREIcazxFvXrUt48dxjrnAtHgP0pGjiJFC-MixEqXL7tL2Wvr4~mlDby0jjrFVkG9s~AKHNcwoKGkjw0qFl6eQDyBp2KWUaNetc5sLrNKN~CHJ4XOIGRv8tDTDVxIqhsaUHtk~Y7dKwkzbPVcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal