Abstract
BACKGROUND: Current AML risk stratification relies on genetic data (cytogenetics and molecular characteristics) and minimal residual disease (MRD) assessment. This approach allows optimizing the post-remission allocation to chemotherapy only or hematopoietic cell transplantation (HCT). We investigated the feasibility and results of a prospective risk-adapted phase II trial of intensive chemotherapy followed or not by autologous or allogeneic HCT based on these criteria.
METHODS: Adult patients with primary AML treated at 15 academic hospitals from the Spanish CETLAM group were included between February 2012 and May 2017. Induction chemotherapy consisted of idarubicin 12 mg/m2 days 1-2-3 IV bolus and cytarabine 200 mg/m2 as continuous infusion days 1 to 7. Consolidation courses were the high-dose cytarabine schedule of the CALGB (3 g/m2 or 1.5 g/m2 if older than 60 years). The number of consolidation courses depending on genetic risk was: three in low-risk category (FR) (CBF, NPM1mut/FLT3-ITDwild or ratio<0.5, CEBPA biallelic mutation); two in intermediate risk category (IR) (intermediate cytogenetics without favorable or unfavorable molecular features (FLT3-ITD, MLL) followed by a HCT (autologous or allogeneic, depending on MRD levels, availability of an HLA matched donor and center preference); and one consolidation in high-risk group (AR) (adverse genetics) followed by alloHCT from the most suitable source available. Patients with FR or IR and high MRD after consolidations (flow cytometry >0.1% or high specific transcripts (RUNX1/RUNX1T1, CBFβ/MYH11, NPM1)) were moved to the AR.
RESULTS: Five hundred patients have been enrolled with a median age (range) of 55 (18-70) years and 53% were male. Revised MRC classification in 476 informative cases was: CBF 11%, intermediate risk cytogenetics 66% (77% of them with a normal karyotype), and poor risk 23%. NPM1 mutation was present in 47% and FLT3-ITD in 30% of patients with intermediate cytogenetics.
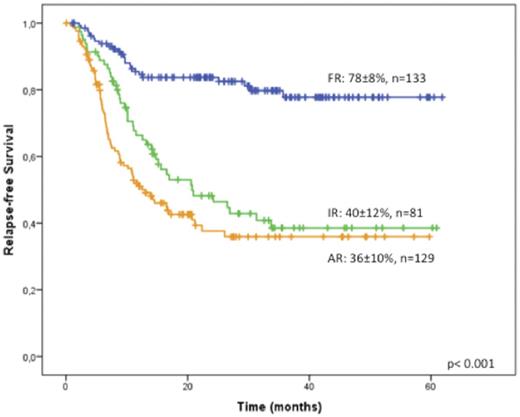
Complete remission (CR) rate was 75% (n=374), 89% of them after a single course of induction CR was 77% in patients up to the age of 60 years and 71% in those older than 60. Induction death occurred in 11% of patients, 8% and 17% the groups up to and above 60 years, respectively; 10% and 13% of patients up to and above 60 years had refractory leukemia and 2% had morphological leukemia-free status. Three hundred and forty-three of the 374 CR patients completed the consolidation phase. The remaining CR patients had early relapse (n=8), death in CR (n=5) or the treatment is ongoing (n=18). Of risk-allocated patients, 133 (38%) were in the genetics-MRD favorable category (FR), 81 (24%) in the IR and 129 (38%) in the AR; in the latter, 94 (73%) of patients received an alloHCT in first CR. Ten patients (4%) moved from FR or IR group to the AR because of high MRD at the end of consolidation. Median follow-up in the surviving patients is 25 months. Overall survival (OS) of the whole series at 4 years is 43±5%; relapse-free survivals are 77±8% in the FR group, 40±12% in the IR and 36±10% in the AR group (Figure 1) due to significantly different cumulative relapse incidence (18%, 43% and 46%, respectively). Remarkably, we confirmed our previous finding that patients with intermediate-risk MRC cytogenetics with NPM1 mutation and FLT3-ITDwild had comparable outcomes to those with NPM1 mutation and an allelic ratio below 0.5. Of the 10 patients who were MRD positive at the end of consolidation (FR and IR), 7 received an alloHCT in 1st CR (5 alive in CR, 1 transplant related mortality and 1 relapsed); the remaining 3 had a morphologic relapse before alloHCT and died. Nine patients from the FR became MRD positive during follow-up and all were allografted: one of them experienced TRM and the remaining 8 are alive in CR.
CONCLUSION
Risk adapted therapy for primary AML based on genetics and MRD is feasible in a cooperative group setting. The proportion of patients in whom the risk of an alloSCT in first CR may be avoided is 38% when considering cytogenetics, molecular findings and MRD information after the end of consolidation phase. MRD assessment at the end of consolidation moved 4% of patients from FR and IR to AR. AlloSCT in AR patients was feasible in most instances, even in the AR group. Despite this, relapses remain above 40% in the intermediate and adverse AML categories and further approaches after transplant such as novel agents and immune therapy deserve investigation.
Ribera: Janssen: Honoraria; Gilead: Honoraria; Celgene: Honoraria; Roche: Honoraria; ARIAD: Research Funding, Speakers Bureau; Incyte: Research Funding, Speakers Bureau; Pfizer: Research Funding, Speakers Bureau; Amgen Inc.: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal