Abstract
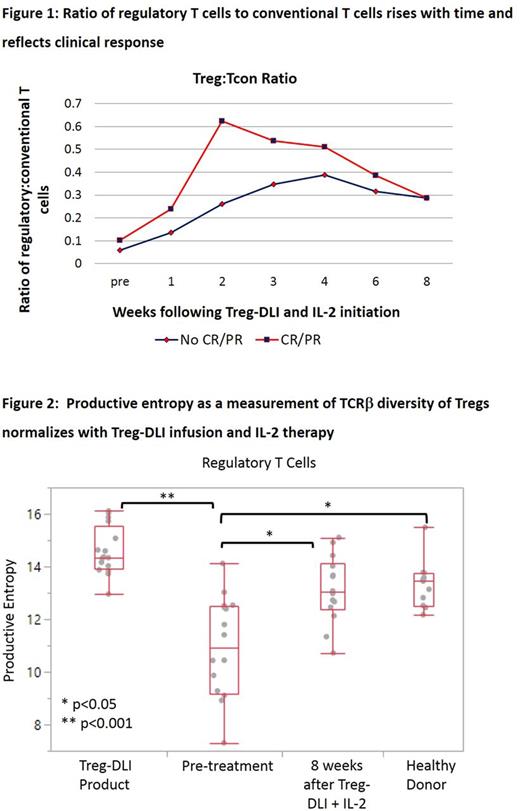
Low-dose daily Interleukin-2 (IL-2) therapy expands regulatory T cells (Treg) in vivo in patients with chronic graft-versus-host disease (cGvHD). Clinical improvement, primarily partial response, is seen in 50-60% of those treated. We hypothesized that infusion of fresh Tregs prior to low dose IL-2 may augment clinical and immune responses. In a Phase 1 study, we administered fresh Treg-enriched lymphocyte infusions (Treg-DLI) obtained from the original stem cell donor, without in vitro stimulation or expansion, followed by daily low-dose IL-2 as therapy for cGvHD. Methods: 24 recipients of 7-8/8 HLA-matched allogeneic hematopoietic stem cell transplants with steroid-refractory cGvHD on stable immune suppression received escalating doses Treg-DLI: 5 each at 0.1, 0.3, or 1x106 cells/kg plus an expansion cohort of 9 at 1x106/kg. Non-mobilized leukapheresis products were CD8/CD19 co-depleted, then positively selected for CD25+ Tregs via the CliniMACS system (Miltenyi). IL-2 was administered at 1x106 U/m2/day for 8 weeks after Treg-DLI infusion. Subjects: The first 22 subjects had a median age of 48 (range, 23-68), median of 3 cGvHD sites (range, 1-6), and median of 2 prior therapies in addition to steroids (range, 0 -5). Four had prior IL-2 exposure. Median time from cGvHD diagnosis to enrollment was 26 months (range, 7- 84). Manufacturing: All Treg-DLI products met release criteria. Median CD8 and CD19 log-depletions were 4.5 and 4.6, respectively. Released products contained a median of 92.6% CD4+CD25++ cells and 88.2% CD4+CD25+CD27low Tregs, a 24.8-fold enrichment (range, 12.7-43.4). At the highest targeted dose level, a median of 0.98x106 cells/kg were infused (range, 0.64-1.0). Safety: In the 21 subjects evaluable for safety, there were no infusional AEs, no DLTs, and no cases of relapse. 2 subjects underwent a 50% dose reduction of IL-2 for Grade 2 flu-like symptoms and injection site irritation, at provider discretion. Grade 3 AEs possibly related to therapy were dyspnea and edema in 1 subject. At median follow-up of 26 months, 17 subjects are alive. 2-year survival is 82% (95% CI, 54-95%). Response: At 8 weeks, 2 of 5 subjects in Cohort 1 (1 partial response (PR), 1 stable disease with minor response (SDMR)), 4 of 5 subjects in Cohort 2 (2 PR, 2 SDMR), and 8 of 11 subjects at the highest cell dose (1 complete response(CR), 3 PR, 4 SDMR) demonstrated clinical benefit. Response rate (CR+PR) was 33%; clinical benefit rate (CR+PR+SDMR) was 67%. 11 subjects opted to continue IL-2, for a median of 14.5 months (range, 3-39). Of 4 subjects previously on IL-2 alone, 2 were responders (CR, PR) and 1 each had mixed response and SDMR, respectively. Interestingly, 3 subjects with SDMR at 8 weeks manifested PR on extended IL-2. No impact of Treg-DLI dose was observed. At 6 months, median prednisone dose was 15mg (range, 5-40), down from 30mg daily at baseline (range, 5-80). Correlative Immunology: Treg counts and the ratio of Treg to conventional T cells (Tcon) rose over time. Higher Treg:Tcon ratios at baseline (p=0.08) and at 2 weeks into therapy (p=0.03) were associated with clinical response (Figure 1). Breadth of diversity within Treg T-cell receptor β (TCRβ) sequences (Shannon's entropy) and selective expansions of individual Treg clones (clonality) were low in cGvHD subjects at baseline, as compared to normal donors (p<0.01) and to Treg-DLI products (p<0.001). Treg TCRβ entropy increased in cGvHD subjects after 8 weeks of therapy (p<0.05), and resulting entropy was comparable to normal donors (n=16, Figure 2). This normalization was independent of clinical response and persisted through 6 months (n=8). Treg TCRβ clonality trended upwards at 8 weeks but did not reach levels seen in normal donors. Entropy and clonality of CD8 and Tcon TCRβ populations in cGvHD subjects were comparable to normal donors and did not change with therapy. Conclusions: GMP-grade manufacture ofCD25+ enriched Treg-DLI is feasible. In combination with low-dose IL-2, Treg-DLI is safe and has clinical efficacy in steroid-refractory cGvHD, including in those with inadequate responses to IL-2 alone. TCRβ population diversity specifically in Tregs is normalized with therapy. The incremental benefit of adding Treg-DLI to therapy with IL-2 alone on clinical responses, normalization of immunologic diversity, and functional suppressive activity, including at timepoints beyond 8 weeks, is the subject of ongoing analyses.
Nikiforow: Kite Therapeutics: Membership on an entity's Board of Directors or advisory committees. Armand: Affimed: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Infinity: Consultancy; Otsuka: Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Sequenta/Adaptive: Research Funding; Genmab: Consultancy; Roche: Research Funding; Tensha: Research Funding; Pfizer: Consultancy, Research Funding; Sigma Tau: Research Funding. Antin: Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Koreth: Millennium Pharmaceuticals: Research Funding; Prometheus Labs: Research Funding; Kadmon Corp: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Amgen Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal