Abstract
Background
Disease recurrence is the principal cause of treatment failure after allogeneic hematopoietic stem cell transplantation (allo-HSCT) for hematologic malignancies. New strategies for maintaining remission are urgently needed. The pilot study suggested that post-transplant therapy with low dose Azacitidine (AZA), a DNA methyltransferase inhibitor, was safe and promising for patients with high-risk hematologic malignancies although the long-term outcome remains to be seen (Cancer. 2010; 116: 5420-31). Gemtuzumab ozogamicin (GO) is a calicheamicin-conjugated antibody to CD33 which exerts anti-leukemic effect in a Syk expression dependent manner and AZA restores Syk expression of leukemic blasts. We previously conducted the pilot study to evaluate the safety and efficacy of post-transplant maintenance therapy with AZA and GO (AZA-GO) (Br J Haematol. 2015; 169: 756-9). Herein, we analyzed the outcome of patients with high-risk hematologic malignancies who received AZA-GO as post-transplant maintenance therapy at our institute.
Methods
Twenty-three adult patients with high-risk hematologic malignancies (21 acute myeloid leukemia, 1 myelodysplastic syndromes excess blast-2, and 1 mixed-phenotype acute leukemia) harboring CD33 positive leukemic blasts were enrolled. AZA-GO was started after sufficient hematological recovery following allo-HSCT. AZA at 30 mg/m2 was administered for 7 days, followed by GO at 3 mg/m2 on day 8. Next cycle was given upon hematological recovery. The maximum number of cycles was 4. To evaluate the impact of AZA-GO on survival, overall survival (OS), disease-free survival (DFS), relapse and non-relapse mortality rates were compared with 69 matched-control patients, who survived more than 30 days after transplant and did not receive any post-transplant maintenance treatment. The matched-control patients were randomly selected from the patient database at our institution, matched for diagnosis, refined disease risk index (R-DRI), and number of transplants.
Results
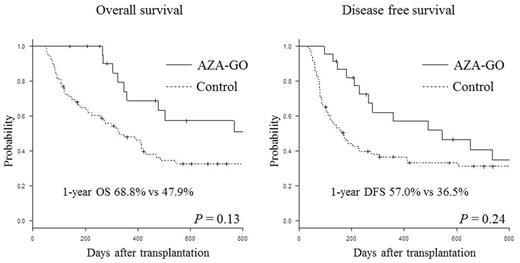
Using R-DRI, patients were stratified into intermediate (n=4), high (n=14), and very high (n=5) risk groups. Median age was 54 years (range, 17 to 67). At transplant, 17 patients did not achieve complete remission. Eight patients harbored adverse cytogenetics and 4 patients received AZA-GO after second HSCT. Eight patients received allo-HSCT from HLA haploidentical donor and 11 patients received reduced-intensity conditioning. Patients received a total of 52 cycles of AZA-GO, starting at a median of 78 days from transplant (range, 24 to 251). The median cycle of treatment was 2. Reasons for stop receiving AZA-GO were relapse (n=6), infections (n=4), thrombocytopenia (n=3), deterioration of GVHD (n=1), elevated creatinine (n=1), and refusal (n=1). The median follow-up of survivors was 526 days (range, 141 to 1627) from allo-HSCT. Grade 4 neutropenia and thrombocytopenia were experienced by 22 of 23 and all patients. Febrile neutropenia occurred in 10 patients. During observation, 13 patients had died and the causes of death included relapse (n=10), infection (n=2) and thrombotic microangiopathy (n=1). One patient developed fatal pneumocystis pneumonia. Of 13 patients suffered from disease recurrence following AZA-GO treatment, 7 patients underwent allo-HSCT as a salvage therapy and none of them developed sinusoidal obstructive disease. During AZA-GO treatment, 3 patients developed grade III acute GVHD. Compared to matched case-control group, the patients who received AZA-GO maintenance showed higher OS (68.8% vs 47.9% at 1 year, p=0.13), DFS (57.0% vs 36.5% at 1 year, p=0.24), lower relapse (32.7% vs 41.3% at 1 1year, p=0.67) and non-relapse mortality rate (10.4% vs 22.2% at 1 year, p=0.16), although the differences were not statistically significant.
Conclusion
AZA-GO maintenance therapy was tolerable in early post-transplant settings and showed acceptable OS and DFS considering nearly three quarters of patients were not in remission at transplant. However, half of the patients developed disease recurrence and there were no significant differences between AZA-GO and control group on their survival and relapse rates. Our current post-transplant strategy requires longer follow-up and further modifications to maintain long-term remission.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal