Abstract
Background: Most relapses occur within 1 year post-transplant and relapse is the major cause of treatment (Tx) failure after allo-SCT for patients (pts) with high-risk AML and MDS. Novel therapeutic strategies are urgently required. The DNA methyltransferase inhibitor, azacitidine (AZA), was shown to increase expression of epigenetically silenced leukemia antigens and induce a CD8+ T-cell response to tumor antigens post-transplant. AZA has also been shown to accelerate Treg reconstitution in murine transplant models, resulting in reduced risk of graft-versus-host disease (GvHD). Thus, AZA maintenance therapy could improve post-transplant outcomes in AML and MDS through epigenetic manipulation of the allo-immune response. Encouraging preliminary data have been reported for subcutaneous (SC) AZA, although challenges with exposure and compliance are limitations of SC administration. CC-486 is a novel oral formulation of AZA that allows prolonged AZA exposure with extended dosing schedules and sustained DNA hypomethylation over the course of the Tx cycle. We investigated the optimal dose and schedule, tolerability, and clinical activity of CC-486 maintenance Tx after allo-SCT.
Methods: Pts who had received allo-SCT for MDS or AML, with myeloablative (MAC) or reduced-intensity (RIC) conditioning and who had a sibling or unrelated donor with ≤1 HLA mismatch, were eligible. Pts were to be in morphological complete remission (CR) at CC-486 Tx initiation (ie, ≤5% bone marrow [BM] blasts) and must have had ANC ≥1.0x109/L and platelets ≥25x109/L. CC-486 Tx was to begin 42-84 days after allo-SCT. In the dose-finding phase, two 7-day schedules (200 mg and 300 mg QD) and two 14-day schedules (200 mg and 150 mg QD) of CC-486 were evaluated, each in 28-day Tx cycles. Tx was to continue for 12 cycles.
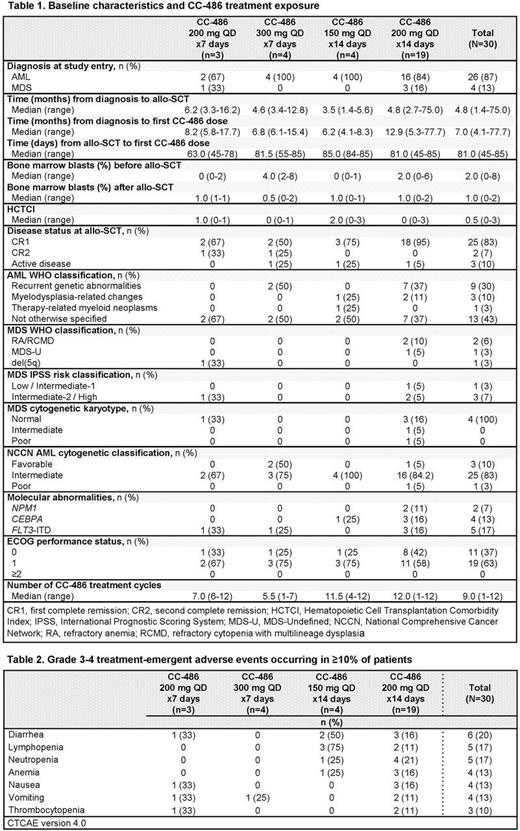
Results: Of 31 pts enrolled, 1 pt did not receive CC-486; thus, the evaluable cohort comprised 30 pts, including 4 pts with MDS and 26 pts with AML (Table 1). Twenty-nine pts were in CR at transplant and 1 pt had ~8% BM blasts on day of transplant. The maximum tolerated dose was not reached. Consequently, the CC-486 200 mg QD 14-day cohort was expanded to include additional pts for further assessment of the clinical activity of this regimen. Median follow-up for all pts was 19.0 months (range 1.0-41.3). Thirteen pts (43%) completed all 12 Tx cycles; of the 17 pts (57%) who discontinued, 6 (20%) did so due to AML or MDS relapse, 5 (17%) withdrew consent, 3 (10%) had a non-GvHD Tx-emergent adverse event (TEAE), and 1 pt each discontinued due to death, development of chronic GvHD, or recurrence of AML involvement in the CNS. The median number of CC-486 cycles delivered was 9 (range 1-12). Ten of 19 pts (53%) in the CC-486 200 mg QD 14-day dosing cohort completed all 12 Tx cycles.
Eight of 30 pts (27%) experienced relapse or progressive disease (PD); 7 relapses occurred within 1 year of study entry, including 3 within the first CC-486 Tx cycle. Median relapse- and progression-free survival (RPFS) for all pts was 34.1 months (95%CI 15.3, 37.8). Rates of relapse/PD were 57% (4/7) for pts in the combined 7-day dosing cohort and 17% (4/23) in the combined 14-day dosing group. In the 14-day dosing cohorts, median RPFS was not reached (NR; 95%CI 10.2, NR), nor was median OS (95%CI 15.1, NR). One-year survival rates in the 7-day and 14-day dosing cohorts were 86% and 81%, respectively, and 1-year RPFS rates were 54% and 72%.
Three pts (10%) experienced grade 3 acute GvHD and no pt had grade 4 acute GvHD. Eight pts experienced chronic GvHD but only 2 cases were considered severe. The most common grade 3-4 TEAEs were gastrointestinal and hematological and did not increase proportionately among dosing regimens (Table 2).
Conclusions: CC-486 maintenance Tx was associated with low rates of relapse and acute or chronic GvHD, with a median RPFS of ~3 years. Extended CC-486 dosing regimens were generally well tolerated, and median OS and RPFS were not reached in the combined 14-day dosing cohort. The CC-486 200 mg 14-day dosing regimen will move forward for further study. Our data are consistent with the hypothesis that post-transplant maintenance therapy may reduce the risk of disease relapse or progression in pts allografted for AML or MDS, and support the hypothesis that CC-486 maintenance may permit epigenetic manipulation of the alloreactive response post-allograft. These findings require confirmation in prospective randomized trials.
de Lima: Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Research Funding. Oran: Celgene: Research Funding; AROG: Research Funding; Astex: Research Funding. Scott: Acceleron: Other: Data and Safety Monitoring Board; Incyte: Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Agios Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees. William: Miragen: Consultancy, Honoraria. Hetzer: Celgene Corporation: Employment, Equity Ownership. Hubbell: Celgene Corporation: Employment, Equity Ownership. Skikne: Celgene Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal