Abstract
Combination chemotherapy with ABVD is widely used as first line therapy for adult classical Hodgkin lymphoma (cHL). However, for patients in the Adolescent and Younger Adult (AYA) age group, there is no consensus regarding preferred upfront therapy and diverse approaches have been used. Given this, we sought to assess the suitability of ABVD for AYA patients by undertaking a comparative analysis of outcomes for AYA patients and other adults treated at a single institution employing an ABVD-based approach.
This study includes ABVD-treated patients with biopsy-proven classical Hodgkin lymphoma diagnosed consecutively between 2006 and 2017 at a single United Kingdom institution. AYA patients were defined as individuals aged 16-26 years at diagnosis. Advanced disease was defined as Ann Arbor stage IIB/III/IV, or stage IA/IB/IIA with adverse features comprising mediastinal bulk or ≥3 involved sites. Early disease was defined as stage IA/IB/IIA in the absence of adverse features. Patients with advanced cHL were treated by intention with 6 cycles of ABVD-based therapy, whilst early stage patients were treated by intention with 3-4 cycles of ABVD with or without involved field radiotherapy (IFRT).
158 patients were included, of whom 69 (44%) were AYA patients aged 16-26 years (median 21 years) and 89 (56%) were other adults aged 27-73 years (median 47 years). 131 (83%) patients were categorised as advanced cHL, comprising 113 with stage IIB/III/IV disease and 17 with stage IA/IB/IIA disease with adverse features; these included 58 AYA patients and 73 other adults. Amongst those with advanced cHL, 50% had an IPI of 2-4 and 10% had an IPI ≥4, with no difference between age groups.
Amongst the 131 patients with advanced disease, 67 completed 6 cycles of ABVD, 5 received 6 cycles of AVD, 42 had ABVD with subsequent de-escalation to AVD to a total of 6 cycles, and 7 received 2-3 cycles of ABVD with subsequent intensification to escalated BEACOPP because of suboptimal interim response. Six also received IFRT. Ten patients failed to complete 6 cycles of therapy, including 7 with treatment-related toxicity and 2 by patient choice; therapy was discontinued due to poor response in 1 patient.
Amongst patients with advanced cHL, complete response to ABVD was achieved in 79%, including 99 by PET criteria and 5 by CT. 17 patients exhibited progression or relapse, 6 of whom were AYA patients. Of these, 15 proceeded to intensive salvage chemotherapy. Four others received salvage therapy for residual disease without progression. To date, 9 patients have undergone high dose therapy and autologous stem cell transplant (SCT) and 6 have received an allogeneic SCT, in one case following prior autograft. In total, 10 patients have died, including 4 from toxicity related to ABVD, 1 from an unrelated cause 5 years after diagnosis, and 5 with progressive cHL; only one AYA patient has died - from advanced cHL refractory to multiple lines of therapy.
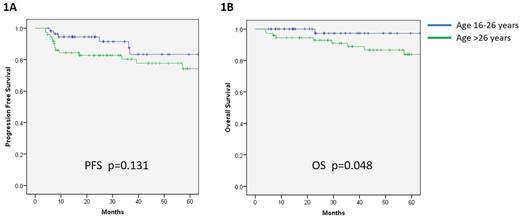
With a median follow-up of 41 months, patients with advanced disease had 3 year progression free survival (PFS) of 85% and 5 year overall survival (OS) of 92%. Notably, there was a trend towards improved PFS for the AYA group in comparison to other adults (92% vs. 80% at 3 years; p=0.131; Figure 1A). Moreover, there was significantly better OS amongst AYA patients (97% vs 89% at 3 years; p=0.048; Figure 1B). Further analysis, including a similar time to progression between age groups, indicates that improved OS observed in AYA patients was predominantly a result of worse treatment tolerability in the older group.
Amongst 27 patients with early stage cHL, 16 completed 3-4 cycles of ABVD, with (n=11) or without (n=5) 30 Grays of IFRT, and 9 others received 6 cycles of ABVD; 2 patients failed to tolerate ABVD. Two patients exhibited disease progression. PFS and OS at 3 years were 96% and 100% respectively. No difference in outcome was observed between age groups for early stage disease.
To our knowledge, this is the first comparative analysis of outcomes for AYA patients treated with an ABVD-based approach for cHL. Amongst all patients with advanced cHL, our reported outcomes compare favourably with published data. Moreover, we show superior outcomes for AYA patients, with significantly improved OS that reflects improved treatment tolerability. In conclusion, our data support the use of ABVD as first line therapy for AYA patients, and furthermore lead us to question the adoption of paediatric approaches for cHL in this age group.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal