Abstract
Introduction: Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy of myeloid progenitor cells. Most patients with AML have a dismal 5-year overall survival rate highlighting the need for novel targets of therapy as well as identification of biology-related factors capable of predicting clinical outcomes with current therapeutic approaches. The cytostatic deoxycytidine analog cytarabine (Ara-C) is currently the most active cytotoxic agent against AML and, thus, represents a key component of primary and salvage chemotherapy in AML. Two recent studies have shown independently that the deoxynucleoside triphosphate (dNTP) triphosphohydrolase SAM domain and HD domain 1 (SAMHD1) determine patient sensitivity to cytarabine, since reduction or loss of SAMHD1 expression dramatically sensitizes AML blasts to cytarabine-induced cytotoxicity (Schneider et al, Nat Med 2017; 23(2):250-255 and Herold et al, Nat Med 2017; 23(2):256-263). Therefore, SAMHD1 expression in AML blasts has been proposed as a novel biomarker for patient response to cytarabine-based chemotherapy as well as a potential therapeutic target.
Methods: The cohort included 100 adult AML patients (56 men, 44 women; median age 56 years) diagnosed and treated at The University of Texas M.D. Anderson Cancer Center from 2008 to 2015. The average follow-up was 19.1 months. All patients belonged to the intermediate risk group and had diploid cytogenetics. Mutation status for FLT3 -ITD, FLT3 -TKD and NPM1 was available for the entire study group. A tissue microarray (TMA) was constructed using duplicate tissue specimens (bone marrow) obtained prior to treatment. SAMHD1 expression was assessed by immunohistochemistry performed on TMA sections using a monoclonal antibody (Bethyl Laboratories, San Antonio, TX) and autostainer system (Ventana). A cutoff of 15% was used to define low- versus high-level SAMHD1 expression. Survival analysis was restricted to patients treated with cytarabine-containing regimens. Overall survival (OS) was defined as time from AML diagnosis to death or last follow-up. Event-free survival (EFS) was defined as days from AML diagnosis to relapse, death, or last follow-up. Patients were censored at the time of hematopoietic stem cell transplantation (HSCT). The association between the proportion of SAMHD1-positive blasts and survival was analyzed using Cox Proportional Hazards Model.
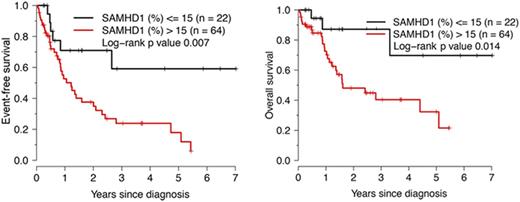
Results: After induction chemotherapy, 83 patients (83%) achieved complete remission (CR) and 1 achieved partial remission (PR). Chemotherapy regimens included cytarabine in 88 patients, and 41 patients underwent stem cell transplantation (HSCT). The percentage of SAMHD1-positive blasts was significantly associated with serum LDH levels (Spearman R=0.38, p=0.0005) and the white blood count (Spearman R=0.24, p=0.03) but not with blast count or CD34 expression in the blast population. Low-level SAMHD1 expression correlated significantly with better OS and EFS in the cohort of 88 patients treated with cytarabine (Figure 1). Survival analysis using Cox regression models and percentage of SAMHD1-positive blasts as a continuous variable showed that SAMHD1 expression is significantly associated with EFS, either unadjusted or adjusted for age, gender, and FLT3 (but not NPM1 ) mutations (all three parameters in the same model, p<0.05, Wald test). As SAMHD1 expression levels did not correlate with response to induction therapy in this AML cohort, the differences in survival may be due to consolidation therapy. No correlation between the percentage of SAMHD1-positive blasts and cytarabine dose was observed.
Conclusions: Low-level SAMHD1 expression (<=15%) in AML blasts at diagnosis identifies a sizeable intermediate-risk patient subset with favorable EFS and OS following induction with cytarabine-containing chemotherapy regimens. As such, SAMHD1 expression assessed by immunohistochemistry may represent a novel predictor of outcome in intermediate-risk AML.
Kantarjian: Novartis: Research Funding; Amgen: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding; Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding. Khoury: Stemline Therapeutics: Research Funding; Pfizer: Research Funding; Kiromics: Research Funding; Angle: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal