Abstract
Acute Graft Versus Host Disease (aGVHD), resulting from an immune mediated attack of donor cells against recipient's healthy tissues remains the bugbear of allogeneic Bone Marrow Transplantation (allo-BMT). Considering the heterogeneous nature of aGVHD, associated high incidence of morbidity and mortality, severe side effects and limited success of current immunosuppressive prophylactic regimens, we were encouraged to develop a novel, safe and easy-to-use intervention for prophylaxis of aGVHD.
Our group, for the first time ever, employed an approach of donor graft modulation with Withaferin A (WA) prior to allogeneic transplantation to decrease the allo-reactivity of donor lymphocytes for mitigating post-transplant aGVHD. WA is a steroidal lactone isolated from the medicinal plant Withania somnifera . Our previous studies have shown the immune-suppressive efficacy of WA on T lymphocytes in vitro {Gambhir et al Toxicol Appl Pharmacol 2015; 289: 297}. Extending those findings, we demonstrate the anti-GVHD potential of WA in a murine model of aGVHD.
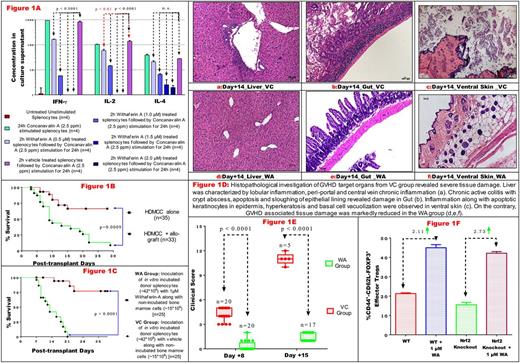
Transient (2h) treatment of lymphocytes of C57Bl/6 mice with 1µM WA inhibited mitogen induced activation, proliferation and cytokine secretion (Figure 1A) in vitro without inducing significant cell death (≤10% at 1µM WA). In order to evaluate graft modulatory potential of WA for alleviating aGVHD in vivo, we validated and characterized a MHC mismatched and High Dose Myeloablative Chemo-Conditioning (HDMCC) based C57BL/6-to-BALB/c aGVHD model earlier reported by Sadeghi et al {Bone Marrow Transplant. 2008;42:807}, except we increased the splenocyte dose to 42*106 per recipient to intensify aGVHD manifestation. In this model, we observed early mortality with a median survival of 9 days (Figure 1B), clinically and histologically relevant characteristics of aGVHD accompanied by cytokine storm in recipients of HDMCC + allo-graft (n=33) compared to HDMCC alone (n=35).
Infusion of donor graft incubated with 1µM WA in vitro for 2 h prior to transplantation abrogated the post-transplant cytokine storm. On day +3, levels of IFN-g, IL-1b, IL-2, IL-6, TNF-a and IL-17 were suppressed by 3.21, 3.18, 1.57, 3.80, 1.70 and 4.32 folds respectively in WA group compared to Vehicle Control (VC) group. Conversely levels of the anti-inflammatory cytokine, IL-10, increased by 1.86 fold in WA group as compared to VC group. The levels of IFN-g, IL-1b and IL-2 continued to be low on day +7 in WA group compared to VC group by 4.53, 1.39 and 1.93 folds respectively.
Further,survival was significantly improved in recipients of allo-graft modulated with WA compared to VC [Hazard Ratio = 11.86 (7.164 to 50.06)] (Figure 1C). GVHD associated tissue damage was also markedly reduced in the WA group as compared to VC group (Figure 1D) . At the same time reconstitution of lymphoid, myeloid and erythroid lineages was also achieved in peripheral blood with complete and stable donor chimerism observed even at day +69. Clinical scoring of aGVHD using minor modification of Hill's method {Blood 1997; 90:3204} showed that mice from VC group had a median score of 11 (10-12) on a scale of 15, with moderate to severe clinical GVHD features on day +15. In contrast, mice from WA group, scored 1 (1-2) with mild clinical GVHD features (Figure 1E).
In order to elucidate the cellular mechanism of action of WA, we studied its effect on T cell differentiation. We found that WA induced T-regulatory (Treg) differentiation in CD4+ T helper cells in vitro . WA is known to activate the transcription factor Nuclear Respiratory Factor-2 (Nrf-2) in a Keap1-independent and Pten/Pi3k/Akt-dependent mechanism. Using Nrf-2 knockout mice, we found that Treg induction by WA was independent of Nrf-2 (Figure 1F) .
In conclusion, we are the first group to show pre-clinical evidence for therapeutic efficacy of WA by in vitro modulation of donor graft prior to transplantation. WA suppressed two causal events in GVHD pathophysiology namely, 'immune-priming' and 'release of myriad cytokines'. In addition, WA also induced Treg differentiation in donor T cells resulting in improved overall survival and reduction in aGVHD associated morbidity without compromising the reconstitution of host immune system. We are currently investigating the GVL effect of WA modulated graft in order to successfully transfer this novel approach to the clinics.
This work was supported by the Department of Biotechnology (6242 P61/RGCB/PMD/DBT/ARMR2015).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal