Abstract
Introduction: The Revised International Staging System (R-ISS) has been widely adopted to prognosticate Multiple Myeloma (MM). As a result, the continued utility of conventional karyotyping, has been called into question.
Methods: A multi-centre study for newly diagnosed MM patients who received novel agent/s at induction from year 1999 at the National University Hospital Singapore, Singapore General Hospital, Alfred Hospital, Melbourne, Australia, was conducted. Conventional karyotype information was categorized based on ploidy as follows: normal, hyperdiploid (48-74 chromosome numbers), non-hyperdiploid (other abnormal karyotype). We evaluated the impact of ploidy on Overall Survival (OS) including a multivariate analysis, taking into account the R-ISS stages, transplant status, age, and novel agent/s used at induction. We also evaluated if it is possible to substitute FISH with conventional karyotyping in identifying high-risk patients. Results were validated in an independent cohort from China.
Results: There were 303 patients evaluable. Ploidy significantly affected OS of patients with R-ISS stage II (N=212; p=0.026), with non-hyperdiploid patients doing worst. For R-ISS stage I patients (N=20), the number of patients in each cytogenetic group was too small to draw any conclusion. Non-hyperdiploid karyotype had no prognostic impact on patients with R-ISS stage III (N=71; p=0.990). In the multivariate analysis, ploidy was significantly associated with OS (p=0.018).
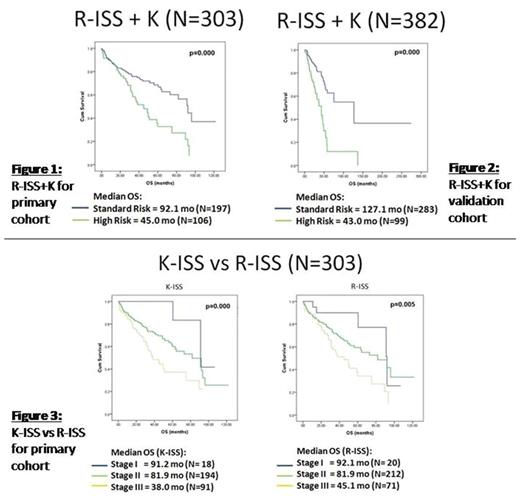
Based on our results, we divided the R-ISS stage II patients into groups with or without non-hyperdiploid karyotype. The R-ISS stage I group and the R-ISS stage II group without non-hyperdiploid karyotype had similar median OS (median OS of 92.1 and 96.0 months respectively; p=0.001). They were grouped together into a standard-risk group. The R-ISS stage II group with non-hyperdiploid karyotype and the R-ISS stage III group had similar OS and were categorized together as a high-risk group (median OS of 45.0 months and 45.1 months respectively; p=0.001). As high-risk myeloma are of most clinical significance, we proposed a new prognostic staging system that incorporates ploidy into the R-ISS. In this R-ISS+K(Karyotype) staging system, patients were divided into standard and high-risk groups.. Patients in the 2 groups had significantly different OS (median OS of 92.1 versus 45.0 months, p=0.000).
We replaced FISH with conventional karyotyping in the K(Karyotype)-ISS staging system, where stage I consisted of patients with ISS stage I with normal LDH and cytogenetics, stage III consisted of patients with ISS stage III with raised LDH or abnormal cytogenetics, and stage II consisted of all others. Of the R-ISS stage III patients, 83.0% were also K-ISS stage III. R-ISS stage II patients who were K-ISS Stage III had median OS of 45.0 months, shorter than K-ISS stage II patients that were R-ISS stage III (median OS of 60.0 months). In this K-ISS staging system, K-ISS stage III patients had median OS of 38.0 months, shorter than the median OS (45.1 months) of the R-ISS stage III group.
These results were validated with an independent cohort of 382 patients from the Institute of Hematology, Peking University, China. The median OS in the R-ISS+K standard risk group was 127.1 months compared to 43.0 months for the high risk group (p=0.000). Similarly, K-ISS stage III patients had median OS of 43.1 months, compared to 46.1 months for the R-ISS stage III patients (p=0.000).
Conclusion: Ploidy by conventional karyotyping is an independent prognostic factor even in the setting of R-ISS. Ploidy can be incorporated into R-ISS staging system to better identify high-risk patients (R-ISS+K). In countries where FISH is not available, conventional cytogenetics can be used instead of FISH (K-ISS) to identify high-risk patients.
Spencer: Amgen: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Chng: Janssen China R&D: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal