Key Points
Introduction of the British HPFH mutation into the fetal globin promoter in a human cell model causes elevated fetal globin expression.
The British HPFH mutation creates a de novo binding site both in vitro and in vivo for the potent erythroid activator KLF1.
Abstract
β-Hemoglobinopathies are among the most common single-locus inherited diseases. In this condition, high fetal hemoglobin (HbF) levels have been found to be beneficial, and boosting HbF expression is seen as an attractive therapy. Naturally occurring mutations in the fetal globin promoter can result in high HbF persisting into adulthood in a benign condition known as hereditary persistence of fetal hemoglobin (HPFH). Individuals with one form of HPFH, British HPFH, carry a T to C substitution at position −198 of the fetal globin gene promoter. These individuals exhibit HbF levels of up to 20%, enough to ameliorate the symptoms of β-hemoglobinopathies. Here, we use clustered regularly interspaced short palindromic repeat–mediated genome editing to introduce the −198 substitution into human erythroid HUDEP-2 cells and show that this mutation is sufficient to substantially elevate expression of HbF. We also examined the molecular mechanism underlying the increase in fetal globin expression. Through a combination of in vitro and in vivo studies, we demonstrate that the mutation creates a de novo binding site for the important erythroid gene activator Krüppel-like factor 1 (KLF1/erythroid KLF). Our results indicate that introducing this single naturally occurring mutation leads to significantly boosted HbF levels.
Introduction
Fetal hemoglobin (HbF) levels are typically downregulated postnatally to ∼1% of total hemoglobin. However, in some individuals, higher levels of HbF persist throughout adulthood. This benign condition, hereditary persistence of fetal hemoglobin (HPFH), has been associated with mutations in regulatory regions of fetal γ-globin. Several HPFH variants have been identified, often in families that presented to the clinic as a result of other disease-causing hemoglobin mutations. Family members who were HPFH carriers tended to experience milder symptoms of disease and, thus, high levels of HbF are considered beneficial.1
A −198T>C mutation in the promoter of the γ-globin gene was first described in 1974 in a large British family2,3 and is known as British-type HPFH (Figure 1A; supplemental Figure 1, available on the Blood Web site). Carriers have HbF levels of ∼5% to 20%. Previously, it was shown that the mutation creates a sequence resembling a specificity protein 1 (SP1)–binding site and that the activator SP1 could bind this site in vitro.4 Accordingly, it was proposed that the ubiquitous SP1 transcription factor is responsible for driving high HbF levels in British HPFH individuals.4,5 It was therefore unexpected that subsequent transactivation assays showed that elevated activity of the mutant promoter is erythroid-specific,4,5 suggesting that an erythroid-specific transcription factor is involved in the overexpression of HbF. Given these results, we reinvestigated and found that the erythroid transcription factor Krüppel-like factor 1 (KLF1)6-8 binds to and drives the expression of the −198T>C promoter.
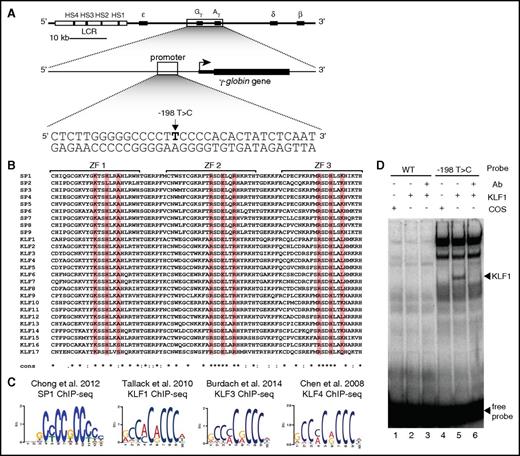
KLF1 binds to and activates the −198T>C γ-globin promoter in vitro. (A) The organization of the human β-globin locus (chromosome 11). The β-like globin genes are indicated by black boxes with their conventional gene symbols indicated above them. The locus-control region (LCR) is represented as a rectangle, with the DNase I hypersensitive sites (HS) within the LCR being represented by black boxes (HS1-4). The British HPFH (−198T>C) mutation in the promoter of the γ-globin gene is also depicted. (B) Aligned protein sequences of the 3 zinc finger (ZF) DNA-binding domains of all members of the SP/KLF family of transcription factors. Highlighted are residues −1, +3, and +6 of each zinc finger, which are responsible for making contact to DNA. (C) In vivo binding motifs of transcription factors SP1,18 KLF1,15 KLF3,16 and KLF417 as determined by ChIP-Seq. (D) EMSA (electrophoretic mobility shift assay) showing that KLF1 can bind in vitro to a −198T>C probe but fails to bind to a WT probe containing the −198 region of the γ-globin promoter. Lanes 1 to 3 contain the WT probe for the −198 site (−209 to −187 bp) and lanes 4 to 6 contain the HPFH −198T>C mutant probe. Lanes 1 and 4 contain nuclear extracts from COS cells transfected with a pcDNA3 empty vector. Lanes 2 to 3 and 5 to 6 contain nuclear extracts from COS cells overexpressing KLF1. Binding of KLF1 to the −198T>C HPFH mutant probe can be observed in lane 5, with a supershift of KLF1 with an anti-KLF1 antibody in lane 6. Ab, antibody; WT, wild type.
KLF1 binds to and activates the −198T>C γ-globin promoter in vitro. (A) The organization of the human β-globin locus (chromosome 11). The β-like globin genes are indicated by black boxes with their conventional gene symbols indicated above them. The locus-control region (LCR) is represented as a rectangle, with the DNase I hypersensitive sites (HS) within the LCR being represented by black boxes (HS1-4). The British HPFH (−198T>C) mutation in the promoter of the γ-globin gene is also depicted. (B) Aligned protein sequences of the 3 zinc finger (ZF) DNA-binding domains of all members of the SP/KLF family of transcription factors. Highlighted are residues −1, +3, and +6 of each zinc finger, which are responsible for making contact to DNA. (C) In vivo binding motifs of transcription factors SP1,18 KLF1,15 KLF3,16 and KLF417 as determined by ChIP-Seq. (D) EMSA (electrophoretic mobility shift assay) showing that KLF1 can bind in vitro to a −198T>C probe but fails to bind to a WT probe containing the −198 region of the γ-globin promoter. Lanes 1 to 3 contain the WT probe for the −198 site (−209 to −187 bp) and lanes 4 to 6 contain the HPFH −198T>C mutant probe. Lanes 1 and 4 contain nuclear extracts from COS cells transfected with a pcDNA3 empty vector. Lanes 2 to 3 and 5 to 6 contain nuclear extracts from COS cells overexpressing KLF1. Binding of KLF1 to the −198T>C HPFH mutant probe can be observed in lane 5, with a supershift of KLF1 with an anti-KLF1 antibody in lane 6. Ab, antibody; WT, wild type.
Study design
In vitro binding assays were performed as previously described.9
HUDEP-2 cells10 were used as a model to study the effects of −198T>C HPFH. Cells were transfected with clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) plasmid px45811 encoding a guide RNA targeting the γ-globin promoter and a single-stranded oligodeoxynucleotide containing the −198T>C mutation. Transfected cells were enriched by fluorescence-activated cell sorting and clonal populations were established.
γ-globin expression was determined by quantitative reverse transcription polymerase chain reaction (qRT-PCR) using primers to amplify the region of interest, and expression was normalized to 18S ribosomal RNA (rRNA). Flow cytometry was performed on permeabilized cells stained with an allophycocyanin (APC)-conjugated HbF antibody.
Chromatin immunoprecipitation (ChIP) was performed as previously described12 using an antibody against KLF1 and control immunoglobulin G.
Hemoglobin high-performance liquid chromatography (HPLC) was performed as previously described.13
Chromatin-conformation-capture (3C) assays were performed as previously described.12
See supplemental Methods for more details.
Results and discussion
SP/KLF transcription factor family members have DNA-binding domains composed of 3 Cys2His2-type zinc fingers that recognize CNCCC motifs where N can be any base but mostly is G or A.14 The DNA-binding domains of all members of the SP/KLF transcription factor family are virtually identical in the 3 residues in each zinc finger that make primary contacts to DNA (Figure 1B). Hence, it is not surprising that the binding sites of different SP/KLF family members are highly similar as determined by ChIP followed by sequencing (ChIP-Seq) experiments15-18 (Figure 1C). Accordingly, we reasoned that, as SP1 has been shown to bind in vitro,4,5 any of the members of the SP/KLF family could potentially interact with the −198T>C γ-globin promoter.
To test this prediction in vitro, we performed EMSAs with erythroid KLF (KLF1/erythroid KLF; Figure 1D) and 12 other KLF proteins (supplemental Figure 2), and assessed binding to the WT and −198T>C γ-globin promoters. All tested KLFs preferentially bound the mutant γ-globin promoter. Next, we investigated the abundance of KLF and SP proteins in erythroid tissues and cell lines because the −198T>C mutation activates γ-globin expression erythroid-specifically.4,5 We found that KLF1 is the most abundantly expressed in differentiated human CD34+ bone marrow cells19 and erythroblast HUDEP-2 cells10 making it a likely candidate to be accountable for elevated HbF levels in British HPFH (supplemental Figure 3).
We then moved to engineer HUDEP-2 cells to stably carry the mutation. HUDEP-2 cells are an attractive recently described model for primary adult erythroblasts, given their expression patterns of globins10 and of the appropriate KLFs/SP proteins (supplemental Figure 3). We introduced targeted substitution mutations into the γ-globin locus using CRISPR/Cas9 genome editing and homology-directed repair to establish clonal cell populations carrying the −198T>C mutation (supplemental Figure 4).
First, we examined messenger RNA (mRNA) expression levels of β-like globins by qRT-PCR (Figure 2A). Clonal WT HUDEP-2 cells express ∼0.5% to 1% γ-globin mRNA [γ/γ+β]. Introducing the −198T>C mutation into a single fetal globin promoter elevated the percentage of γ-globin mRNA to 4% to 6%. Accordingly, we also observed that the percentage of HbF-immunostaining cells (“F cells”) increased in cells carrying the −198T>C mutation (Figure 2B).
KLF1 binds to and activates the −198T>C γ-globin promoter in HUDEP-2 cells. (A) mRNA expression levels of γ-globin displayed as a percentage of γ- and β-globin [γ/γ+β]. mRNA levels were determined by qRT-PCR and cycle threshold (Ct) values were normalized to rRNA levels of 18S. Primer efficiencies were confirmed to be equivalent. Shown are clonal HUDEP-2 WT cells (n = 4) and 2 clones (n = 2 for #15 and n = 3 for #17) each carrying 1 allele with the −198T>C mutation. γ-globin mRNA levels are significantly higher in cells carrying the −198T>C mutation (**P < .01). (B) Flow cytometry analysis of HbF levels in clonal WT HUDEP-2 cells (n = 3) and a clonal HUDEP-2 cell population that is heterozygous for the −198T>C mutation (n = 3). Representatively shown is the median of 3 experiments (± standard deviation [SD]). Cells were permeabilized and then stained with APC-conjugated HbF antibody. The amount of cells expressing high HbF (“F cells”) was determined by flow cytometry. The same gating strategy was applied for WT HUDEP-2 cells and −198T>C HUDEP-2 cells using FlowJo software. (C) mRNA expression levels of γ-globin displayed as a percentage of γ- and β-globin [γ/γ+β]. mRNA levels were determined by qRT-PCR and Ct values were normalized to rRNA levels of 18S. Shown are parental HUDEP-2(ΔGγ)WT cells and HUDEP-2(ΔGγ) cells homozygous for the −198T>C mutation. γ-globin mRNA levels are significantly higher in cells carrying the −198T>C mutation (***P < .0001) (D) Flow cytometry analysis of HbF levels in HUDEP-2(ΔGγ)WT and HUDEP-2(ΔGγ)−198T>C cells. Representatively shown is the median of 3 experiments (±SD). Cells were permeabilized and then stained with APC-conjugated HbF antibody. The amount of cells expressing high HbF (“F cells”) was determined by flow cytometry. (E) HPLC traces depicting hemoglobin production in HUDEP-2(ΔGγ)WT and 3 clonal (ΔGγ)−198T>C cell populations. Highlighted in red is the peak for HbF. Percentages are HbF over total hemoglobin (HbF and HbA0). (F) ChIP-qPCR analysis of the relative enrichment of KLF1 at various genomic loci in HUDEP-2(ΔGγ)WT (n = 2) and −198T>C cells (n = 4). The tested genomic loci were the γ-globin promoter, the β-globin promoter, and the VEGFA promoter (−ctrl). The SP1 promoter served as a positive control (+ctrl) for successful pulldown with the respective antibody and all values were normalized to enrichment at the SP1 promoter. Enrichment of the γ-globin promoter after KLF1 pulldown is significantly higher in HUDEP-2(ΔGγ)−198T>C cells whereas enrichment of the β-globin promoter is significantly lower (**P < .01). (G) ChIP-qPCR analysis of the relative enrichment of KLF1 at genomic loci in 5-day differentiated HUDEP-2(ΔGγ)WT (n = 3) and −198T>C cells (n = 3). Again, KLF1 enrichment is significantly higher (*P < .05) at the γ-globin promoter but lower (**P < .01) at the β-globin promoter. (H) 3C assay measuring locus-wide crosslinking frequencies in HUDEP-2(ΔGγ)WT (n = 3) and 3 clonal −198T>C cell populations. A schematic of the human β-globin locus is shown on top of the graph. The x-axis indicates distances in kilobases (kb) from the ε-globin gene. Vertical lines represent HindIII restriction sites. The dark gray bar denotes the anchor HindIII fragment containing hypersensitive site 3. Light gray bars denote analyzed HindIII fragments. Shown is mean ± standard error of the mean (s.e.m.). (I) Proposed model of molecular mechanism in British HPFH. In WT adult erythroid cells, KLF1 drives the expression of adult β-globin via the promoter (top panel). The presence of the −198T>C mutation (bottom panel) allows binding of KLF1 to the fetal γ-globin promoter and leads to upregulation of γ-globin expression through recruitment of the LCR. In these cells, binding of KLF1 to the β-globin promoter and β-globin transcript levels are reduced. n.s., not significant; SSC, side scatter.
KLF1 binds to and activates the −198T>C γ-globin promoter in HUDEP-2 cells. (A) mRNA expression levels of γ-globin displayed as a percentage of γ- and β-globin [γ/γ+β]. mRNA levels were determined by qRT-PCR and cycle threshold (Ct) values were normalized to rRNA levels of 18S. Primer efficiencies were confirmed to be equivalent. Shown are clonal HUDEP-2 WT cells (n = 4) and 2 clones (n = 2 for #15 and n = 3 for #17) each carrying 1 allele with the −198T>C mutation. γ-globin mRNA levels are significantly higher in cells carrying the −198T>C mutation (**P < .01). (B) Flow cytometry analysis of HbF levels in clonal WT HUDEP-2 cells (n = 3) and a clonal HUDEP-2 cell population that is heterozygous for the −198T>C mutation (n = 3). Representatively shown is the median of 3 experiments (± standard deviation [SD]). Cells were permeabilized and then stained with APC-conjugated HbF antibody. The amount of cells expressing high HbF (“F cells”) was determined by flow cytometry. The same gating strategy was applied for WT HUDEP-2 cells and −198T>C HUDEP-2 cells using FlowJo software. (C) mRNA expression levels of γ-globin displayed as a percentage of γ- and β-globin [γ/γ+β]. mRNA levels were determined by qRT-PCR and Ct values were normalized to rRNA levels of 18S. Shown are parental HUDEP-2(ΔGγ)WT cells and HUDEP-2(ΔGγ) cells homozygous for the −198T>C mutation. γ-globin mRNA levels are significantly higher in cells carrying the −198T>C mutation (***P < .0001) (D) Flow cytometry analysis of HbF levels in HUDEP-2(ΔGγ)WT and HUDEP-2(ΔGγ)−198T>C cells. Representatively shown is the median of 3 experiments (±SD). Cells were permeabilized and then stained with APC-conjugated HbF antibody. The amount of cells expressing high HbF (“F cells”) was determined by flow cytometry. (E) HPLC traces depicting hemoglobin production in HUDEP-2(ΔGγ)WT and 3 clonal (ΔGγ)−198T>C cell populations. Highlighted in red is the peak for HbF. Percentages are HbF over total hemoglobin (HbF and HbA0). (F) ChIP-qPCR analysis of the relative enrichment of KLF1 at various genomic loci in HUDEP-2(ΔGγ)WT (n = 2) and −198T>C cells (n = 4). The tested genomic loci were the γ-globin promoter, the β-globin promoter, and the VEGFA promoter (−ctrl). The SP1 promoter served as a positive control (+ctrl) for successful pulldown with the respective antibody and all values were normalized to enrichment at the SP1 promoter. Enrichment of the γ-globin promoter after KLF1 pulldown is significantly higher in HUDEP-2(ΔGγ)−198T>C cells whereas enrichment of the β-globin promoter is significantly lower (**P < .01). (G) ChIP-qPCR analysis of the relative enrichment of KLF1 at genomic loci in 5-day differentiated HUDEP-2(ΔGγ)WT (n = 3) and −198T>C cells (n = 3). Again, KLF1 enrichment is significantly higher (*P < .05) at the γ-globin promoter but lower (**P < .01) at the β-globin promoter. (H) 3C assay measuring locus-wide crosslinking frequencies in HUDEP-2(ΔGγ)WT (n = 3) and 3 clonal −198T>C cell populations. A schematic of the human β-globin locus is shown on top of the graph. The x-axis indicates distances in kilobases (kb) from the ε-globin gene. Vertical lines represent HindIII restriction sites. The dark gray bar denotes the anchor HindIII fragment containing hypersensitive site 3. Light gray bars denote analyzed HindIII fragments. Shown is mean ± standard error of the mean (s.e.m.). (I) Proposed model of molecular mechanism in British HPFH. In WT adult erythroid cells, KLF1 drives the expression of adult β-globin via the promoter (top panel). The presence of the −198T>C mutation (bottom panel) allows binding of KLF1 to the fetal γ-globin promoter and leads to upregulation of γ-globin expression through recruitment of the LCR. In these cells, binding of KLF1 to the β-globin promoter and β-globin transcript levels are reduced. n.s., not significant; SSC, side scatter.
After confirming that the −198T>C mutation reproduces an HPFH phenotype in HUDEP-2 cells, we investigated the binding of transcription factors to the −198T>C γ-globin promoter. We first sought to generate homozygous HUDEP-2 −198T>C cells. As it proved difficult to reproducibly target all 4 copies of the γ-globin genes, we introduced an intermediate step and first engineered a parental cell line carrying only 1 γ-globin gene and promoter on each allele (HUDEP-2(ΔGγ)WT; supplemental Figure 5). We then introduced the −198T>C mutation into these cells homozygously, applying the same strategy as outlined previously (HUDEP-2(ΔGγ)−198T>C). We found that γ-globin mRNA levels were strongly elevated compared with the parental cells and that the amount of F cells increased substantially in clonal HUDEP-2(ΔGγ)−198T>C cells (Figure 2C-D). We also performed HPLC for hemoglobin. In HUDEP-2(ΔGγ)WT cells, only adult hemoglobin was detected whereas HbF made up 52% to 77% in cells with −198T>C (Figure 2E). Western blots confirmed this finding and HbF repressor levels (BCL11A/ZBTB7A) were unchanged (supplemental Figure 6).
We then performed KLF1 ChIP and saw consistently higher enrichment of the γ-globin promoter in HUDEP-2(ΔGγ)−198T>C cells compared with the WT (Figure 2F). This effect was augmented when we performed the KLF1 ChIP in 5-day differentiated HUDEP-2(ΔGγ)WT and −198T>C cells (Figure 2G). To ensure that the antibody is specific for KLF1, we also carried out ChIP experiments across a range of loci using KLF1 knockout and WT HUDEP-2 clones. In KLF1 knockout cells, we observed only background binding, equivalent to that obtained with control immunoglobulin G, thereby validating the specificity of the antibody (supplemental Figure 7). Interestingly, we also observed that binding of KLF1 to the β-globin promoter was significantly reduced in HUDEP-2(ΔGγ)−198T>C cells, suggesting that KLF1 may switch from activating β-globin in WT7,20 to driving γ-globin expression in −198T>C cells. Hence, we then looked at interaction frequencies of the LCR with the globin promoters by 3C. We observed that the LCR indeed interacts more frequently with the γ-globin promoter and less often with the β-globin promoter in HUDEP-2(ΔGγ)−198T>C compared with WT cells (Figure 2H).
These findings support our hypothesis that the −198T>C mutation creates a functional binding site for the erythroid activator KLF1 and that de novo binding of KLF1 contributes to the upregulation of γ-globin expression in British HPFH by shifting the LCR toward the fetal globin promoters (Figure 2I). This is consistent with the commonly accepted model of the LCR interacting competitively with the globin promoters. Although present in appropriate levels, BCL11A and ZBTB7A clearly do not silence the HbF expression at the −198T>C promoter, suggesting that de novo binding of KLF1 overrides the developmentally determined gene expression program of globins. This is analogous to what we12 and others21 have seen when other transcription factors that induce chromatin looping are targeted to the γ-globin promoter.
Several approaches have been proposed to reactivate the expression of HbF in adult life, including targeting the HbF repressor BCL11A22 and its regulatory elements23,24 and forced chromatin looping mediated by an artificial transcription factor.21 If the efficiency of targeting is sufficient,18 then introducing naturally occurring mutations associated with HPFH could represent an alternative approach.
Beyond the potential therapeutic aspects, our work is of interest as it shows that the −198T>C mutation generates a de novo binding site. An important insight gained from genome-wide association studies is that cis-regulatory mutations are commonly associated with genetic disease, and are as abundant as coding variants.25 Mutations will typically either disrupt a transcription factor binding site or create a new site. Because a range of deletions, insertions, or substitutions can disrupt elements, but only precise changes can create new elements, one might expect that disruptions are more common than de novo sites. However, the first 2 HPFH mutations we studied, −198T>C and the previously reported −175T>C,12 create de novo sites for erythroid-specific transactivators. Similarly, a de novo GATA1 site can profoundly affect the expression of genes within the α-globin locus.26 It may be that disruptions are less prevalent than expected because of redundancy observed between multiple cis-acting elements27 but de novo sites are in fact more common than initially anticipated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CRISPR/Cas9 genome-editing plasmid px458 was a gift from Feng Zhang, Broad Institute, Cambridge, MA (Addgene plasmid # 48138). The authors thank Laura Norton, Alister Funnell, and Richard Pearson of UNSW Sydney (Sydney, NSW, Australia) for valuable discussions and reagents. The authors also acknowledge Helene Lebhar and the UNSW Recombinant Products Facility for assistance in the analysis of hemoglobin variants.
This work was supported by funding from the Australian National Health and Medical Research Council (APP1098391) (M.C.). B.W. was supported by a University International Postgraduate Award. G.E.M. was supported by an Australian Postgraduate Award.
Authorship
Contribution: B.W. performed most of the experiments; G.E.M. performed EMSA with KLF1; R.K. and Y.N. provided HUDEP-2 cells; K.G.R.Q. and M.C. supervised the study; B.W., K.G.R.Q., and M.C. designed experiments and wrote the manuscript; and all authors have read and approved the contents of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Merlin Crossley, School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW 2052, Australia; e-mail: m.crossley@unsw.edu.au.

![Figure 2. KLF1 binds to and activates the −198T>C γ-globin promoter in HUDEP-2 cells. (A) mRNA expression levels of γ-globin displayed as a percentage of γ- and β-globin [γ/γ+β]. mRNA levels were determined by qRT-PCR and cycle threshold (Ct) values were normalized to rRNA levels of 18S. Primer efficiencies were confirmed to be equivalent. Shown are clonal HUDEP-2 WT cells (n = 4) and 2 clones (n = 2 for #15 and n = 3 for #17) each carrying 1 allele with the −198T>C mutation. γ-globin mRNA levels are significantly higher in cells carrying the −198T>C mutation (**P < .01). (B) Flow cytometry analysis of HbF levels in clonal WT HUDEP-2 cells (n = 3) and a clonal HUDEP-2 cell population that is heterozygous for the −198T>C mutation (n = 3). Representatively shown is the median of 3 experiments (± standard deviation [SD]). Cells were permeabilized and then stained with APC-conjugated HbF antibody. The amount of cells expressing high HbF (“F cells”) was determined by flow cytometry. The same gating strategy was applied for WT HUDEP-2 cells and −198T>C HUDEP-2 cells using FlowJo software. (C) mRNA expression levels of γ-globin displayed as a percentage of γ- and β-globin [γ/γ+β]. mRNA levels were determined by qRT-PCR and Ct values were normalized to rRNA levels of 18S. Shown are parental HUDEP-2(ΔGγ)WT cells and HUDEP-2(ΔGγ) cells homozygous for the −198T>C mutation. γ-globin mRNA levels are significantly higher in cells carrying the −198T>C mutation (***P < .0001) (D) Flow cytometry analysis of HbF levels in HUDEP-2(ΔGγ)WT and HUDEP-2(ΔGγ)−198T>C cells. Representatively shown is the median of 3 experiments (±SD). Cells were permeabilized and then stained with APC-conjugated HbF antibody. The amount of cells expressing high HbF (“F cells”) was determined by flow cytometry. (E) HPLC traces depicting hemoglobin production in HUDEP-2(ΔGγ)WT and 3 clonal (ΔGγ)−198T>C cell populations. Highlighted in red is the peak for HbF. Percentages are HbF over total hemoglobin (HbF and HbA0). (F) ChIP-qPCR analysis of the relative enrichment of KLF1 at various genomic loci in HUDEP-2(ΔGγ)WT (n = 2) and −198T>C cells (n = 4). The tested genomic loci were the γ-globin promoter, the β-globin promoter, and the VEGFA promoter (−ctrl). The SP1 promoter served as a positive control (+ctrl) for successful pulldown with the respective antibody and all values were normalized to enrichment at the SP1 promoter. Enrichment of the γ-globin promoter after KLF1 pulldown is significantly higher in HUDEP-2(ΔGγ)−198T>C cells whereas enrichment of the β-globin promoter is significantly lower (**P < .01). (G) ChIP-qPCR analysis of the relative enrichment of KLF1 at genomic loci in 5-day differentiated HUDEP-2(ΔGγ)WT (n = 3) and −198T>C cells (n = 3). Again, KLF1 enrichment is significantly higher (*P < .05) at the γ-globin promoter but lower (**P < .01) at the β-globin promoter. (H) 3C assay measuring locus-wide crosslinking frequencies in HUDEP-2(ΔGγ)WT (n = 3) and 3 clonal −198T>C cell populations. A schematic of the human β-globin locus is shown on top of the graph. The x-axis indicates distances in kilobases (kb) from the ε-globin gene. Vertical lines represent HindIII restriction sites. The dark gray bar denotes the anchor HindIII fragment containing hypersensitive site 3. Light gray bars denote analyzed HindIII fragments. Shown is mean ± standard error of the mean (s.e.m.). (I) Proposed model of molecular mechanism in British HPFH. In WT adult erythroid cells, KLF1 drives the expression of adult β-globin via the promoter (top panel). The presence of the −198T>C mutation (bottom panel) allows binding of KLF1 to the fetal γ-globin promoter and leads to upregulation of γ-globin expression through recruitment of the LCR. In these cells, binding of KLF1 to the β-globin promoter and β-globin transcript levels are reduced. n.s., not significant; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/6/10.1182_blood-2017-02-767400/4/m_blood767400f2.jpeg?Expires=1770095100&Signature=RBdMpmm4FfKHfRCXmOPTTSW97J6RnwfkgafDMEABWVgX8mgXlsaEY-ixclDTw2ifZSR7znDyX7rfkGzOZw0snSHBtwaMz10W5ZGbdhF~sbBgJDrBvP62gudrcCXHd0LNkk9swJBQQilE7W2DJDKmJlcsZ1IPwMNpsaz5hbFG4Hu2sEoiySGjRa4WEIIprSMwuGdrhoQ3bAvNFlBn-5hn8ncj6oq5YAVCjLhUVycXPlXG0OgWhyF0tdDqMBdGAXu2z8zBAfb9uT5xebJybllXrFzR1L7AlNEuHuUpiHV6h5tv0lcIsoQxW15ZOqFYyNMtohT2pnfYzLZtILTx5RWWsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal