In this issue of Blood, Stein et al1 demonstrate the feasibility and clinical efficacy of targeted inhibition of mutant isocitrate dehydrogenase 2 (IDH2) in patients with acute myeloid leukemia (AML). In a related paper, Amatangelo et al2 investigate mechanisms of response in samples derived from the same patients.
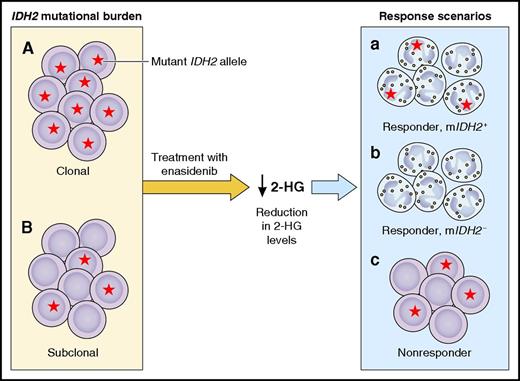
Response dynamics in IDH2 mutant AML patients treated with enasidenib. Mutational burden in IDH2 mutant (mIDH2) AML can vary between the clonal (A) or subclonal (B) presence of a mIDH2 allele. In virtually all patients, treatment with enasidenib strongly reduced 2-HG plasma levels. In spite of this near-universal effect on 2-HG, various response scenarios were observed. Some patients showed evidence of a clinical response, schematically depicted in response scenarios a and b as differentiation of blasts to mature neutrophils. Of those patients, some remained mIDH2+ (a), whereas others became mIDH2− (b). A third group of patients did not attain a clinical response, schematically depicted as a continued predominance of blasts that are partly or completely mIDH2+ (c). This simplified figure only indicates the extreme situations of either CR or complete resistance. Professional illustration by Patrick Lane, ScEYEnce Studios.
Response dynamics in IDH2 mutant AML patients treated with enasidenib. Mutational burden in IDH2 mutant (mIDH2) AML can vary between the clonal (A) or subclonal (B) presence of a mIDH2 allele. In virtually all patients, treatment with enasidenib strongly reduced 2-HG plasma levels. In spite of this near-universal effect on 2-HG, various response scenarios were observed. Some patients showed evidence of a clinical response, schematically depicted in response scenarios a and b as differentiation of blasts to mature neutrophils. Of those patients, some remained mIDH2+ (a), whereas others became mIDH2− (b). A third group of patients did not attain a clinical response, schematically depicted as a continued predominance of blasts that are partly or completely mIDH2+ (c). This simplified figure only indicates the extreme situations of either CR or complete resistance. Professional illustration by Patrick Lane, ScEYEnce Studios.
A block in myeloid differentiation is a hallmark of AML.3 Undifferentiated blast cells with enhanced self-renewal capabilities can accumulate in the bone marrow. When these undifferentiated cells acquire properties that provide a proliferative advantage, AML may arise. It has long been hypothesized that overcoming the differentiation block in AML would be an important component of improved treatment regimens. The best example of this principle is all-trans retinoic acid (ATRA), which revolutionized treatment of patients with acute promyelocytic leukemia (APL) 30 years ago.4 For other subtypes of AML, however, the identification of viable means of differentiation therapy has proven to be more challenging.
Based on our current unprecedented progress in describing the genetic and epigenetic landscape of AML, we know that aberrant differentiation is frequently, at least in part, driven by epigenetic deregulation.5,6 Examples include mutations in genes involved in DNA methylation and modification of histone marks. Mutations in IDH2 are observed in 10% to 12% of patients with AML, while another 10% of patients carry mutations in the related IDH1 gene. Whereas the metabolic enzymes isocitrate dehydrogenase 1 (IDH1) and IDH2 do not directly regulate epigenetic processes, many of the functional consequences of mutations in these proteins converge around epigenetic regulation. Mutant IDH enzymes acquire a neomorphic function that allows them to convert their physiological metabolite, α-ketoglutarate (α-KG), into the aberrant metabolite R-2-hydroxyglutarate (R-2-HG).7 R-2-HG, in turn, is thought to play a central role in leukemogenesis by acting as a competitive inhibitor of α-KG–dependent enzymes. These include epigenetic regulators important for differentiation, such as the DNA 5-methylcytosine hydroxylase TET2 and histone lysine demethylases.8 As a result, by producing excess amounts of R-2-HG, mutant IDH enzymes impair the maturation of hematopoietic stem/progenitor cells and contribute to leukemia development. Accordingly, the specific pharmacological inhibition of mutant IDH reduces the levels of R-2-HG and restores cellular differentiation in vitro and in vivo.9-12
It is unknown whether IDH inhibitors are tolerated in patients and whether these compounds induce clinical responses. Stein et al provide for the first time answers to these questions, and, excitingly, both answers are positive. In a phase 1/2 clinical trial in patients with IDH2 mutant myeloid malignancies, they investigated the tolerability and clinical efficacy of the oral selective mutant IDH2 inhibitor enasidenib (AG-221) as monotherapy. Enasidenib was safe and generally well tolerated. Of note, one of the recurrent, generally manageable toxicities encountered was a differentiation syndrome, which is in some ways reminiscent of symptoms observed in APL patients treated with ATRA. Stein et al investigated clinical responses in patients with relapsed/refractory (R/R) AML (n = 176). Treatment with enasidenib induced an overall response in 40.3% of patients, almost half of which achieved a complete remission (CR). In a subset of patients, enasidenib served as a bridge to allogeneic stem cell transplantation. Median overall survival of the patients achieving a CR was 19.7 months. These are encouraging numbers in the subgroup of R/R AML, which has a notoriously dismal prognosis when treated with conventional modalities.
In spite of the relatively high response rates, not all patients experienced a clinical benefit. While the studies validate total plasma 2-HG levels (used here as a proxy for R-2-HG) as a potent pharmocodynamic marker for on-target effects, they also illustrate that 2-HG levels are not useful as a predictor of response. In fact, dramatic reductions of 2-HG were observed in almost all patients on treatment, irrespective of clinical outcome (see figure). Thus, a reduction of 2-HG levels is not sufficient for clinical response. Similarly, IDH2 mutational burden was not predictive, as responses were seen in patients with minor IDH2 mutant subclones as well as in patients with fully clonal IDH2 mutations.2 Amatangelo et al performed additional genetic studies to identify alternative predictive biomarkers. No single mutations emerged, and clinical responses were seen across the complete spectrum of cytogenetic and molecular risk groups. The authors did identify a higher number of accompanying mutations and RAS pathway mutations as factors associated with a lower likelihood of response.
Does enasidenib induce differentiation of leukemic blasts in AML patients? Morphological and immunophenotypical assessments were in support of this. Furthermore, Amatangelo et al found that in many patients, the allelic burden of IDH2 mutations did not decrease in sequential samples, frequently not even in patients with clear clinical responses. This implies that upon treatment, the mutant alleles were not cleared, but instead remained present within mature cells (see figure, response a). Indeed, in some cases, the investigators demonstrated the presence of functional IDH2 mutant neutrophils after treatment. Interestingly, in the subset of patients achieving a morphological CR, there were 2 different scenarios. In most of those patients, the allelic burden of IDH2 mutations did not decrease over time, consistent with the situation outlined above. A few patients with a morphological CR, however, became completely IDH2 mutation negative (see figure, response b), at least based on the digital PCR technique used. These findings indicate heterogeneity in response dynamics among IDH2 mutant patients. They raise the important question whether enasidenib can target leukemic stem cells (LSCs), the holy grail of AML therapy. One explanation for the 2 scenarios observed could be that enasidenib generally targets the bulk of AML cells and not LSCs, but in some patients, exhaustion of leukemia propagating cells can take place upon prolonged exposure. This important issue requires further research.
Other questions emerge from these studies. What are the long-term effects of enasidenib? Will clonal selection during treatment occur? Intriguingly, some patients who achieved a CR had an IDH2 mutation in only a relatively small subclone at study entry. This implies that leukemic blasts lacking an IDH2 mutation (see figure panel B, cells without an asterisk) responded to enasidenib treatment as well (see figure, response a or b). Possible explanations for this unexpected finding that will need to be investigated in follow-up studies include cell nonautonomous mechanisms of IDH2 mutations and/or direct effects of enasidenib on IDH2 wild-type cells.
Together, the results of these studies argue for the further clinical exploration of IDH inhibitors. It is expected that for more powerful responses, differentiation-based IDH2 inhibition will need to be combined with orthogonal treatment modalities, such as standard chemotherapy or other types of mechanism-based targeted therapy.12 Obviously, in the next phase, enasidenib-based regimens should be compared head-to-head to standard regimens in a randomized controlled fashion. Such studies, and studies with other IDH2 and IDH1 inhibitors, will address the full role of IDH inhibition in AML treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal