To the editor:
Peripheral T-cell lymphomas (PTCLs) represent a group of rare hematological cancers of mature T-cell or natural killer cell origin accounting for 10% to 15% of all lymphomas.1 Although many patients have poor outcomes, some achieve long-term survival.2,3 Thus, identifying prognostic biomarkers is important to facilitate risk-adapted therapy.
Chromosomal rearrangements are critical in the molecular pathogenesis of nearly all types of hematologic neoplasms.4 The best-characterized rearrangements in PTCLs involve the anaplastic lymphoma kinase (ALK) gene, which result in oncogenic ALK fusion proteins and define a specific World Health Organization (WHO) subtype (ALK-positive anaplastic large cell lymphoma [ALCL], representing 6% to 8% of PTCLs).2,5,6 ALK also serves as a prognostic marker: patients with ALK-positive ALCLs have better outcomes than those with ALK-negative ALCLs or other nodal PTCLs, including angioimmunoblastic T-cell lymphoma (AITL) and PTCL, not otherwise specified (NOS).2,7 The molecular pathogenesis of other PTCLs is being elucidated.8 Recurrent chromosomal rearrangements involving the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangements) and the TP53 homolog TP63 on 3q28 recently were reported in ALK-negative ALCL.9-12 DUSP22 rearrangements are associated with decreased expression of dual-specificity phosphatase-22, an enzyme that regulates mitogen-activated protein kinase signaling.10,13,14 A retrospective study found that DUSP22 rearrangements were associated with favorable outcomes in ALK-negative ALCL.12 TP63 rearrangements encoding p63 fusion proteins were associated with aggressive clinical behavior and poor outcomes.11,12
In the present study, we evaluated the prognostic impact of DUSP22 and TP63 rearrangements in diagnostic tumor biopsy specimens from a population-based Danish cohort of 138 patients with nodal PTCLs. Formalin-fixed, paraffin-embedded tumors from patients diagnosed with PTCL between 1984 and 2011 were retrieved from the pathology departments at 3 Danish tertiary referral centers (Aarhus, Herlev, and Odense University Hospitals). Specimens were pretreatment biopsy specimens from newly diagnosed PTCL patients aged ≥16 years for whom pre- and posttreatment clinical information was available. Clinicopathological data were obtained from the population-based database of the Danish National Lymphoma Registry, supplemented by medical records. Cases were reviewed and classified by expert hematopathologists, and re-reviewed to assure concordance with current WHO criteria.15 The study was approved by The Central Denmark Region Committees on Health Research Ethics (record 1-10-72-392-12), the Danish Data Protection Agency (record 1-16-02-26-11), and the institutional review boards at the participating institutions and was conducted in accordance with the Declaration of Helsinki.
Fluorescence in situ hybridization was performed on sections of previously constructed tissue microarrays16 using break-apart probes for the DUSP22-IRF4 and TP63 loci and a dual-fusion probe for TBL1XR1/TP63 fusion (inv(3)(q26q28)) as previously described.10,11 DUSP22 and TP63 rearrangements were assessed in a blinded fashion without knowledge of PTCL subtype, clinical course, or outcome. Differences between groups of categorical and continuous variables were tested using Fisher’s exact test, Wilcoxon rank-sum test, and Kruskal-Wallis equality-of-populations rank test as appropriate. Overall survival (OS) was defined as the time from diagnosis to last follow-up or death from any cause. Association of genetic subtype with OS was assessed using Kaplan-Meier curves and Cox proportional hazards models. Significant differences were defined as P < .05. Statistical analyses were performed using STATA IC 11 (StataCorp, College Station, TX).
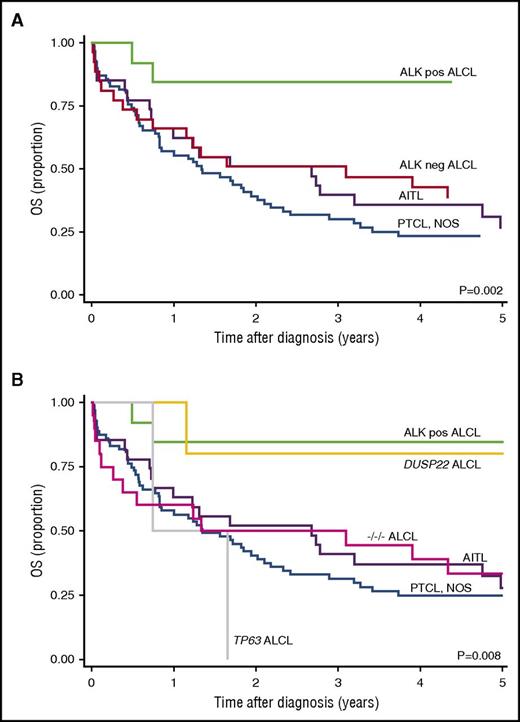
Of 169 PTCLs evaluated, 138 had sufficient material for DUSP22 and TP63 assessment. Subtypes, demographics, and clinical characteristics are shown in Table 1. Median age was 60 years (range, 16-92 years) with a male to female ratio of 1.6:1. Most patients (86%) received curative-intent anthracycline-containing combination chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP], CHOP + etoposide, or other CHOP-like regimens). ALK-positive ALCL patients were younger (P = .07) and had better outcomes (5-year OS, 85%; 95% confidence interval [CI], 51% to 96%; P < .002) than patients with PTCL, NOS, AITL, and ALK-negative ALCL (Figure 1A).
Clinical characteristics of the study cohort (N = 138)
| Characteristic . | PTCL, NOS (n = 71) . | AITL (n = 27) . | ALK-positive ALCL (n = 13) . | ALK-negative ALCL . | All PTCL (n = 138) . | ||
|---|---|---|---|---|---|---|---|
| DUSP22 (n = 5) . | TP63 (n = 2) . | −/−/− (n = 20)* . | |||||
| Age, y | |||||||
| Median | 61 | 64 | 46 | 49 | 44 | 61 | 60 |
| Range | 20-92 | 43-89 | 19-85 | 35-85 | 16-71 | 34-89 | 16-92 |
| Sex | |||||||
| Female | 31 | 11 | 4 | 0 | 2 | 6 | 54 |
| Male | 40 | 16 | 9 | 5 | 0 | 14 | 84 |
| Ann Arbor stage | |||||||
| I | 11 | 2 | 1 | 0 | 1 | 8 | 23 |
| II | 6 | 2 | 1 | 1 | 0 | 5 | 15 |
| III | 20 | 12 | 6 | 1 | 1 | 2 | 42 |
| IV | 29 | 11 | 5 | 3 | 0 | 5 | 53 |
| Unclear | 5 | 0 | 0 | 0 | 0 | 0 | 5 |
| Performance status | |||||||
| 0-1 | 52 | 17 | 10 | 3 | 2 | 14 | 98 |
| ≥2 | 18 | 10 | 3 | 2 | 0 | 6 | 39 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Extranodal sites | |||||||
| ≤1 | 56 | 20 | 9 | 3 | 2 | 16 | 106 |
| >1 | 15 | 7 | 4 | 2 | 0 | 4 | 32 |
| Skin involvement | |||||||
| No | 60 | 23 | 10 | 2 | 2 | 17 | 114 |
| Yes | 2 | 2 | 3 | 3 | 0 | 1 | 11 |
| Unknown | 9 | 2 | 0 | 0 | 0 | 2 | 13 |
| LDH > upper normal limit | |||||||
| Yes | 43 | 17 | 4 | 2 | 1 | 9 | 76 |
| No | 25 | 10 | 9 | 3 | 1 | 9 | 57 |
| Missing | 3 | 0 | 0 | 0 | 0 | 2 | 5 |
| IPI | |||||||
| 1-2 | 32 | 12 | 10 | 3 | 2 | 12 | 71 |
| 3-4 | 26 | 14 | 3 | 2 | 0 | 4 | 49 |
| Missing | 13 | 1 | 0 | 0 | 0 | 4 | 18 |
| Initial treatment | |||||||
| CHOP/CHOP-like | 59 | 23 | 13 | 5 | 2 | 16 | 118 |
| Other | 12 | 4 | 0 | 0 | 0 | 4 | 20 |
| HDT/ASCT | 10 | 8 | 2 | 3 | 0 | 0 | 23 |
| Response | |||||||
| CR | 36 | 18 | 9 | 5 | 0 | 10 | 79 |
| PR | 10 | 2 | 1 | 0 | 0 | 4 | 17 |
| NC | 2 | 1 | 0 | 0 | 0 | 1 | 4 |
| PD | 7 | 2 | 1 | 0 | 2 | 1 | 12 |
| Not evaluated | 8 | 4 | 2 | 0 | 0 | 3 | 17 |
| No treatment | 8 | 0 | 0 | 0 | 0 | 1 | 9 |
| Outcome | |||||||
| 5-y OS, % | 26 | 28 | 85 | 80 | 0 | 33 | 35 |
| 95% CI | 16-37 | 12-46 | 51-96 | 20-97 | — | 14-54 | 27-43 |
| Characteristic . | PTCL, NOS (n = 71) . | AITL (n = 27) . | ALK-positive ALCL (n = 13) . | ALK-negative ALCL . | All PTCL (n = 138) . | ||
|---|---|---|---|---|---|---|---|
| DUSP22 (n = 5) . | TP63 (n = 2) . | −/−/− (n = 20)* . | |||||
| Age, y | |||||||
| Median | 61 | 64 | 46 | 49 | 44 | 61 | 60 |
| Range | 20-92 | 43-89 | 19-85 | 35-85 | 16-71 | 34-89 | 16-92 |
| Sex | |||||||
| Female | 31 | 11 | 4 | 0 | 2 | 6 | 54 |
| Male | 40 | 16 | 9 | 5 | 0 | 14 | 84 |
| Ann Arbor stage | |||||||
| I | 11 | 2 | 1 | 0 | 1 | 8 | 23 |
| II | 6 | 2 | 1 | 1 | 0 | 5 | 15 |
| III | 20 | 12 | 6 | 1 | 1 | 2 | 42 |
| IV | 29 | 11 | 5 | 3 | 0 | 5 | 53 |
| Unclear | 5 | 0 | 0 | 0 | 0 | 0 | 5 |
| Performance status | |||||||
| 0-1 | 52 | 17 | 10 | 3 | 2 | 14 | 98 |
| ≥2 | 18 | 10 | 3 | 2 | 0 | 6 | 39 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Extranodal sites | |||||||
| ≤1 | 56 | 20 | 9 | 3 | 2 | 16 | 106 |
| >1 | 15 | 7 | 4 | 2 | 0 | 4 | 32 |
| Skin involvement | |||||||
| No | 60 | 23 | 10 | 2 | 2 | 17 | 114 |
| Yes | 2 | 2 | 3 | 3 | 0 | 1 | 11 |
| Unknown | 9 | 2 | 0 | 0 | 0 | 2 | 13 |
| LDH > upper normal limit | |||||||
| Yes | 43 | 17 | 4 | 2 | 1 | 9 | 76 |
| No | 25 | 10 | 9 | 3 | 1 | 9 | 57 |
| Missing | 3 | 0 | 0 | 0 | 0 | 2 | 5 |
| IPI | |||||||
| 1-2 | 32 | 12 | 10 | 3 | 2 | 12 | 71 |
| 3-4 | 26 | 14 | 3 | 2 | 0 | 4 | 49 |
| Missing | 13 | 1 | 0 | 0 | 0 | 4 | 18 |
| Initial treatment | |||||||
| CHOP/CHOP-like | 59 | 23 | 13 | 5 | 2 | 16 | 118 |
| Other | 12 | 4 | 0 | 0 | 0 | 4 | 20 |
| HDT/ASCT | 10 | 8 | 2 | 3 | 0 | 0 | 23 |
| Response | |||||||
| CR | 36 | 18 | 9 | 5 | 0 | 10 | 79 |
| PR | 10 | 2 | 1 | 0 | 0 | 4 | 17 |
| NC | 2 | 1 | 0 | 0 | 0 | 1 | 4 |
| PD | 7 | 2 | 1 | 0 | 2 | 1 | 12 |
| Not evaluated | 8 | 4 | 2 | 0 | 0 | 3 | 17 |
| No treatment | 8 | 0 | 0 | 0 | 0 | 1 | 9 |
| Outcome | |||||||
| 5-y OS, % | 26 | 28 | 85 | 80 | 0 | 33 | 35 |
| 95% CI | 16-37 | 12-46 | 51-96 | 20-97 | — | 14-54 | 27-43 |
—, Not evaluable. CR, complete response; HDT, high-dose therapy; IPI, International Prognostic Index; LDH, lactate dehydrogenase; NC, no change; PD, progressive disease; PR, partial response.
−/−/−, triple-negative ALCL.
Outcomes in patients with PTCL. (A) Five-year OS rates (Kaplan-Meier estimates) stratified by PTCL subtype and ALK status only (current WHO classification). (B) Five-year OS rates with ALK-negative ALCL stratified by genetic subtype. ALK-pos ALCL, anaplastic lymphoma kinase–positive anaplastic large cell lymphoma; ALK-neg DUSP22 ALCL, anaplastic lymphoma kinase–negative DUSP22-rearranged anaplastic large cell lymphoma; ALK-neg TP63 ALCL, anaplastic lymphoma kinase–negative TP63-rearranged anaplastic large cell lymphoma; −/−/− ALCL, triple-negative anaplastic large cell lymphoma (negative for ALK, DUSP22, and TP63).
Outcomes in patients with PTCL. (A) Five-year OS rates (Kaplan-Meier estimates) stratified by PTCL subtype and ALK status only (current WHO classification). (B) Five-year OS rates with ALK-negative ALCL stratified by genetic subtype. ALK-pos ALCL, anaplastic lymphoma kinase–positive anaplastic large cell lymphoma; ALK-neg DUSP22 ALCL, anaplastic lymphoma kinase–negative DUSP22-rearranged anaplastic large cell lymphoma; ALK-neg TP63 ALCL, anaplastic lymphoma kinase–negative TP63-rearranged anaplastic large cell lymphoma; −/−/− ALCL, triple-negative anaplastic large cell lymphoma (negative for ALK, DUSP22, and TP63).
Of 27 ALK-negative ALCLs, 5 (19%) had DUSP22 rearrangements, 2 (7%) had TP63 rearrangements, and 20 (74%) were triple negative (ie, in addition to being ALK negative, they lacked DUSP22 and TP63 rearrangements). One PTCL, NOS with some ALCL-like features had rearrangements of both DUSP22 and TP63 (supplemental Figure 1, available on the Blood Web site). No DUSP22 or TP63 rearrangement was detected in the remaining cases of PTCL, NOS or in any ALK-positive ALCL or AITL.
Differences in OS among PTCL subtypes were significant when ALK-negative ALCLs were stratified by genetics (P = .008; Figure 1B) and varied widely among genetic subgroups of ALCL (P = .01; supplemental Figure 2). Patients with DUSP22-rearranged ALK-negative ALCL had a 5-year OS rate of 80% (95% CI, 20% to 97%), similar to that of ALK-positive ALCL patients (85%; P = .85). Both ALCL patients with TP63 rearrangements died with progressive disease within 2 years of initial diagnosis, despite favorable International Prognostic Index scores. Patients with triple-negative ALCLs were slightly older (median age, 61 years) than patients with ALK-positive, DUSP22-rearranged, and TP63-rearranged ALCLs (46, 49, and 44 years, respectively, P = .1). They had an intermediate 5-year OS rate (33%; 95% CI, 14% to 54%), which was poorer than ALK-positive ALCL and DUSP22-rearranged ALCL, better than TP63-rearranged ALCL, and similar to ALK-negative ALCL as a whole (5-year OS, 40%; 95% CI, 22% to 58%). All 5 patients with DUSP22-rearranged ALCLs achieved complete remission; the only death was from a non–lymphoma-related cause. Both ALCL patients with TP63 rearrangements and the PTCL, NOS patient with DUSP22 and TP63 rearrangements had primary refractory disease and died of lymphoma progression.
This study is the first to independently confirm that DUSP22 and TP63 rearrangements predict outcome in ALK-negative ALCL. It is also the first study to examine the prognostic significance of these rearrangements in a prospectively accrued, population-based patient cohort representing all major categories of PTCL. In concordance with Parrilla Castellar et al,12 DUSP22-rearranged ALCL had an excellent prognosis similar to that of ALK-positive ALCL. TP63 rearrangements were rare and were associated with chemotherapy refractoriness and poor outcome. Triple-negative ALCLs had intermediate OS rates inferior to those of DUSP22-rearranged ALCLs and superior to those of TP63-rearranged cases. The PTCL, NOS with rearrangements of both DUSP22 and TP63 had a poor clinical outcome. A previously reported primary cutaneous ALCL with both rearrangements also had an aggressive clinical course11 ; co-occurrence of DUSP22 and TP63 rearrangements has not been reported in systemic ALCL.12 These findings suggest that cases with both rearrangements may behave more similarly to cases with TP63 rearrangements than to those with DUSP22 rearrangements. The current series also confirms the absence of DUSP22 and TP63 rearrangements in ALK-positive ALCL and adds novel information regarding the absence of the rearrangements in AITL and most cases of PTCL, NOS. The genetic profile of triple-negative ALCLs and the distribution of other genetic findings in ALCL (including alterations of ERBB4, ROS1, TYK2, and VAV1 and genes in the JAK-STAT3 pathway17-20 ) merit further study.
This study represents an important first independent validation of the relative frequencies and prognostic significance of DUSP22 and TP63 rearrangements in ALCL. The overall number of cases in each genetic subtype was limited, and thus this series lacked power to formally test survival differences among the ALK-negative genetic subgroups. However, the striking reproducibility of the outcomes after genetic stratification provides a compelling rationale to study the implications of genetic status for clinical management further in larger population-based cohorts and in the clinical trial setting. For example, it has been suggested that DUSP22-rearranged ALCLs may not derive additional benefit from autologous stem cell transplantation in first remission.12 Accordingly, the predictive significance of DUSP22 and TP63 rearrangements currently is being evaluated further in the setting of a large Nordic PTCL trial of upfront autologous stem cell transplantation (NLG-T-01).21
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Kristina Lauridsen (Laboratory of Molecular Pathology, Aarhus University Hospital), Lindsay B. Emanuel (Department of Health Sciences Research, Mayo Clinic), and Kathryn E. Pearce (Department of Laboratory Medicine and Pathology, Mayo Clinic) for excellent technical and logistic assistance.
This work was supported by grants from the Institute of Clinical Medicine, Aarhus University/Aarhus University Hospital, The Karen Elise Jensen Foundation, The A. P. Møller and Chastine Mc-Kinney Møller Foundation for General Purposes, The Eva and Henry Frænkel Foundation, The Mimi & Victor Larsens Fund, the National Institutes of Health, National Cancer Institute (R01 CA177734 and P50 CA97274), and the Department of Laboratory Medicine and Pathology, Mayo Clinic.
Contribution: M.B.P., F.d’A. and A.L.F. drafted the manuscript; F.d’A. and A.L.F. designed the study. R.P.K., P.P.B., I.M.L., and C.A.S. scored and interpreted fluorescence in situ hybridization testing; R.L.B. and N.N.B. contributed to data interpretation; S.J.H.-D., K.B., P.N., M.B.M., and T.S. provided samples and provided pathology review; M.B.P. performed statistical analysis; and all authors approved the final manuscript.
Conflict-of-interest disclosure: A.L.F. and R.P.K. are inventors of technology discussed in this manuscript for which Mayo Clinic holds an unlicensed patent (A.L.F.) or has submitted a patent application (A.L.F. and R.P.K.). The remaining authors declare no competing financial interests.
Correspondence: Andrew L. Feldman, Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: feldman.andrew@mayo.edu; and Francesco d’Amore, Department of Hematology, Aarhus University Hospital, DK-8000 Aarhus C, Denmark; e-mail: frandamo@rm.dk.
References
Author notes
F.d’A. and A.L.F. contributed equally to this study.