Key Points
KRASG12D expression in mouse lung myeloid cells induces pulmonary LCH-like neoplasms.
KRASG12D-induced LCH-like neoplasms are sensitive to in vivo treatment with 3-hydroxy-3-methylglutaryl coenzyme A inhibitor atorvastatin.
Abstract
Langerhans cell histiocytosis (LCH) is a rare histiocytic neoplasm associated with somatic mutations in the genes involved in the RAF/MEK/extracellular signal-regulated kinase (ERK) signaling pathway. Recently, oncogenic mutations in NRAS/KRAS, upstream regulators of the RAF/MEK/ERK pathway, have been reported in pulmonary, but not in nonpulmonary, LCH cases, suggesting organ-specific contribution of oncogenic RAS to LCH pathogenesis. Using a mouse model expressing KRASG12D in the lung by nasal delivery of adenoviral Cre recombinase (Cre), here we show that KRASG12D expression in lung-resident myeloid cells induces pulmonary LCH-like neoplasms composed of pathogenic CD11chighF4/80+CD207+ cells. The pathogenic cells were mitotically inactive, but proliferating precursors were detected in primary cultures of lung tissue. These precursors were derived, at least in part, from CD11cdimCD11bintGr1− lung-resident monocytic cells transformed by KRASG12D. In contrast, BRAFV600E expression induced by the same method failed to develop LCH-like neoplasms, suggesting that each oncogene may initiate pulmonary LCH by transforming different types of lung-resident myeloid cells. In vivo treatment of the KRASG12D-induced LCH-like mouse with the cholesterol-lowering drug atorvastatin ameliorated the pathology, implicating statins as potential therapeutics against a subset of pulmonary LCH.
Introduction
Langerhans cell histiocytosis (LCH) is a rare disorder characterized by abnormal growth of CD1a+S100+ histiocytes that share some characteristics with epidermal Langerhans cells (LCs).1,2 Multiple organs, including the skin, bone, liver, and lung, are affected in LCH either as a multisystem or single-organ disease, and hematopoietic organ involvement has been shown to be a feature of high-risk diseases.1 Pulmonary involvement occurs either in isolation or as part of multisystem diseases, though >85% cases of pulmonary LCH have been reported as single-organ involvement.3
Recent discoveries of somatic mutations in the genes involved in the RAS/RAF/MEK/extracellular signal-regulated kinase (ERK) pathway have unveiled that LCH pathogenesis is driven by neoplastic transformation associated with deregulated ERK pathway activation.4-9 The BRAFT1799A mutation, leading to BRAFV600E oncoprotein expression, is most common in LCH,4 whereas other genes in the ERK pathway, including ARAF5 and MAP2K1,6,7 have been also reported as mutational targets. Furthermore, NRAS8 and KRAS9 mutations, leading to NRASQ61K/R and KRASG12V/A oncoprotein expression, respectively, have been identified uniquely in pulmonary LCH cases, suggesting organ-specific contribution of oncogenic RAS to the pathogenesis of LCH.
Both LCs and lesional LCH cells express Langerin (also known as CD207), a C-type lectin that contributes to the formation of Birbeck granules in LCs/LCH cells.10,11 However, Langerin expression is not restricted to LCs: it is also detected in subsets of dendritic cells (DCs), including CD103+ DCs.12 A previous study utilizing genetically engineered mouse (GEM) models demonstrated that BRAFV600E expression in CD11c+ or Langerin+ DC-lineage cells is sufficient to drive LCH-like pathology in mice, proposing that LCH could originate from CD11c+ DC precursors or Langerin+ mature DCs.13 However, it is currently unclear whether Langerin promoter-driven Cre recombinase (Cre) expression used in the model13 drives BRAFV600E-induced neoplastic transformation of LCs or Langerin+ DCs.
In contrast to conventional DCs (including Langerin+ DCs) developing in a FLT3 (also known as CD135)-dependent manner,12 mouse epidermal LCs, defined by their CD11c+Langerin+CD103−F4/80+ phenotype,12 arise largely from embryonic/fetal precursors, including yolk-sac–derived myeloid progenitors14,15 and fetal liver monocytes,16 in a CSF1R (also known as MCSFR or CD115)-dependent manner.17,18 LC precursors distributed in the embryonic epidermis express CD11c and Langerin immediately after birth,19 suggesting that BRAFV600E induction in the GEM models above13 could start during the first week of life at the latest. Importantly, the majority of human pulmonary LCH cases are adult onset, in contrast to multisystem LCH that is predominantly diagnosed during childhood.20 Therefore, animal models recapitulating the adult-onset pathology in the lung are preferred in order to study the pathogenesis of pulmonary LCH that could be considerably different from multisystem LCH.
Conditional knockin mice expressing KRASG12D following Cre expression have been previously reported and extensively used to study the pathophysiological consequences of oncogenic RAS expression in different tissues including the lung. Although KRASG12D expression in the lung following exposure to an adenoviral vector encoding Cre under control of the cytomegalovirus (CMV) promoter (AdCMVCre)21 has been previously used to study lung adenocarcinoma, somewhat unexpectedly, we found that this model developed mixed neoplasms with hematopoietic and epithelial components. Our investigations confirm that the hematopoietic pathology is driven by KRASG12D expression in specific myeloid population(s) in the lung, recapitulating some aspects of pulmonary LCH. Using this model as a therapeutic platform, we found that the pulmonary LCH-like disease is sensitive to in vivo treatment with atorvastatin, suggesting therapeutic potential of statins for the treatment of RAS-driven pulmonary LCH.
Materials and methods
Animals
Animal experiments were performed under UK Home Office License authority. KrasLSL-G12D, BrafLSL-V600E, and their recombined alleles were genotyped as described.22,23 Nasal delivery of adenoviral vectors was performed as described.23 Atorvastatin (10 mg/kg; Sigma) dissolved in phosphate-buffered saline was orally administrated once a day for 5 weeks, starting at 5 weeks after adenoviral induction. Lung tissues were processed for hematoxylin-and-eosin (H&E) staining and immunohistochemistry (IHC) as described.22 Antibodies used for IHC are described in the supplemental Methods (available on the Blood Web site).
Immunofluorescence, immunoblotting, flow cytometry, and cell sorting
Paraformaldehyde-fixed, Triton-X–permeabilized cells were stained for Mac2, and blocked for endogenous biotin as described.24 Then, the cells were stained with anti-CD207-biotin antibody (1:100; eBioscience), followed by Alexa Fluor 488–conjugated streptavidin (Invitrogen) staining (1:2000). Images were obtained and processed as described.24 Protein lysates were prepared by solving frozen tissues into 1× sodium dodecyl sulfate sample buffer or cultured cells into NP40 buffer, and analyzed by western blotting as described.25,26 Cell surface markers were analyzed by flow cytometry as described.24 For in vitro 5-bromo-2′-deoxyuridine (BrdU) incorporation, cells labeled with 10 μM BrdU for 18 hours were analyzed as described.24 For cell sorting, lung tissues digested by 30-minute collagenase/DNase treatment were stained for cell surface markers as described,24 and sorted using FACSAria II (BD Biosciences). Antibodies used are described in the supplemental Methods.
Cell cultures
Cell cultures were performed essentially as described.24 Detailed methods are described in the supplemental Methods.
In vitro adenoviral infection and limiting dilution culture
Cells from KRASLSL-G12D lungs were plated at 2 × 105 per well to 10 × 105 per well, and infected with adenoviral vectors at a multiplicity of infection equal to (MOI) 100. After 1 week of infection, media containing the virus were removed, and the cultures were maintained in Dulbecco modified Eagle medium/10% fetal calf serum with half media changes every 3 to 5 days without disturbing nonadherent cells. Limiting dilution analysis was performed using ELDA software.27
Coculture of sorted myeloid cells with lung fibroblast feeders
A total of 1 × 105 sorted myeloid cells were infected with adenovirus vectors at MOI = 100 in Dulbecco modified Eagle medium/10% fetal calf serum for 24 hours, and transferred onto fibroblast feeders established by serial passages of wild-type lung cells. On day 7, media containing the virus were removed, and the cultures were maintained as described for limiting dilution culture.
Statistics
Comparison between any 2 groups was performed by the unpaired Student t test. χ2 tests were used to statistically evaluate the differences in limiting dilution cultures.
Results
Lung-restricted KRASG12D expression induces accumulation of CD11c+F4/80+ cells
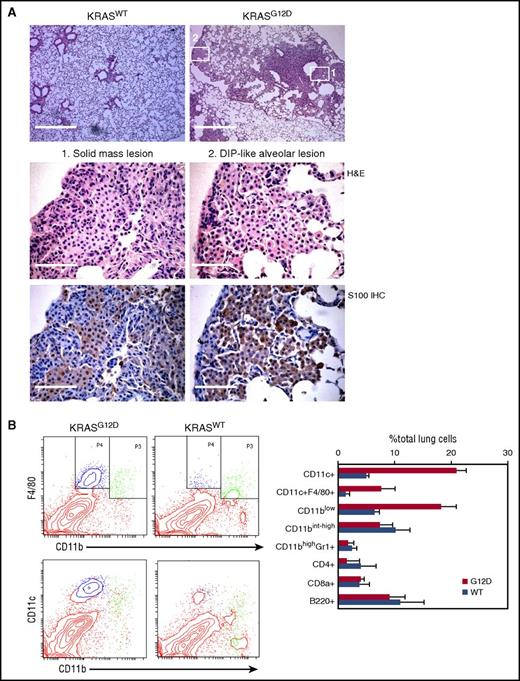
Nasal inhalation of AdCMVCre into KRASLSL-G12D/+ mice has been widely exploited to investigate lung carcinogenic processes. Although AdCMVCre could induce oncogene expression in any type of cell when successfully infected, nasal inhalation of 105 plaque-forming units (pfu) AdCMVCre preferentially promotes epithelial transformation in this model.21 To investigate whether higher viral doses could induce neoplastic transformation of other cell types, we administrated 3 × 107 pfu AdCMVCre into KRASLSL-G12D/+ mice by nasal inhalation (referred to as AdCre/KRASG12D mice hereafter), and AdCre/KRASG12D mice exhibiting severe respiratory symptoms at 4 to 6 months following nasal inhalation were analyzed histologically. As expected, a number of solid mass lesions containing epithelial components were observed, but these lesions were frequently intertwined with round cells with a less eosinophilic cytoplasm (Figure 1A). In addition, distal alveolar spaces were occasionally filled with similar round cells, mimicking the pathology of desquamative interstitial pneumonia (DIP) (Figure 1A). The round cells in both solid and DIP-like lesions were positive for S100, a marker for LCH2 (Figure 1A).
Characterization of hematopoietic cells accumulating in the AdCre/KRASG12Dlung. (A) Histological analysis of the AdCre/KRASG12D lung. Top, low magnification images of H&E-stained lung sections from AdCre/KRASWT and AdCre/KRASG12D mice at 4 months following nasal delivery of AdCre. Scale bar, 0.5 mm. Middle and bottom, high magnification images of H&E staining (middle) and S100 IHC (bottom), corresponding to the areas indicated in the top right image of the AdCre/KRASG12D lung (left, solid mass lesion; right, DIP-like alveolar lesion). Scale bar, 62.5 μm. (B) Flow cytometry analysis of hematopoietic compartments in the AdCre/KRASG12D lung in comparison with AdCre/KRASWT controls. Representative contour plots for CD11b-F4/80 and CD11b-CD11c staining are indicated in the left, and the right bar graph summarizes the percentage of each hematopoietic population in total lung cells. The distribution of CD11blowF4/80+ (blue) and CD11bint-highF4/80+ (green) cells are indicated in CD11b-CD11c plots in the bottom left. The data in the right bar graph represent mean + standard deviation (SD) (n = 3). WT, wild type.
Characterization of hematopoietic cells accumulating in the AdCre/KRASG12Dlung. (A) Histological analysis of the AdCre/KRASG12D lung. Top, low magnification images of H&E-stained lung sections from AdCre/KRASWT and AdCre/KRASG12D mice at 4 months following nasal delivery of AdCre. Scale bar, 0.5 mm. Middle and bottom, high magnification images of H&E staining (middle) and S100 IHC (bottom), corresponding to the areas indicated in the top right image of the AdCre/KRASG12D lung (left, solid mass lesion; right, DIP-like alveolar lesion). Scale bar, 62.5 μm. (B) Flow cytometry analysis of hematopoietic compartments in the AdCre/KRASG12D lung in comparison with AdCre/KRASWT controls. Representative contour plots for CD11b-F4/80 and CD11b-CD11c staining are indicated in the left, and the right bar graph summarizes the percentage of each hematopoietic population in total lung cells. The distribution of CD11blowF4/80+ (blue) and CD11bint-highF4/80+ (green) cells are indicated in CD11b-CD11c plots in the bottom left. The data in the right bar graph represent mean + standard deviation (SD) (n = 3). WT, wild type.
Because the morphology of the round cells observed in AdCre/KRASG12D lungs was suggestive of their hematopoietic origin, we analyzed hematopoietic compartments in the AdCre/KRASG12D lungs using flow cytometry. Compared with KRAS+/+ mice nasally delivered with AdCMVCre (referred to as AdCre/KRASWT mice hereafter), the percentage of CD11blowCD11c+ cells was robustly increased in the AdCre/KRASG12D lung (Figure 1B). Interestingly, the CD11blowCD11c+ population in the AdCre/KRASG12D lung included the majority of the cells expressing a macrophage/LC marker F4/80 (Figure 1B blue in the plot), whereas most F4/80+ cells in the AdCre/KRASWT lung were detected in CD11bintCD11clow/− cells (Figure 1B green in the plot). Collectively, the hematopoietic pathology was characterized by aberrant accumulation of CD11blowCD11c+F4/80+ cells.
CD11c+ cells in the AdCre/KRASG12D lung are oncogene-driven neoplastic cells expressing CD207
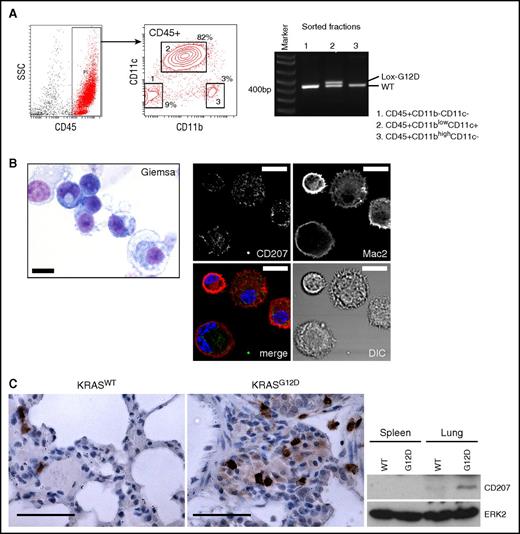
Because nasal delivery of AdCMVCre into KRASLSL-G12D lungs induces epithelial neoplasms,21 it is unclear whether the accumulated CD11c+ cells could represent KRASG12D-transformed lung myeloid cells, or occur as a reactive response of nonneoplastic CD11c+ cells (not expressing KRASG12D) to epithelial neoplasms. To distinguish these possibilities, we sorted lung CD45+ hematopoietic subpopulations according to their CD11b/CD11c profiles, and investigated KRASG12D recombination in each subpopulation. KRAS recombination was clearly detected in the CD45+CD11blowCD11c+ population (Figure 2A), indicating that the accumulation of CD11c+ cells was driven by their KRASG12D expression.
CD207 expression in lung CD11c+cells driven by KRASG12D. (A) Polymerase chain reaction (PCR) detection of the recombined KRASG12D (Lox-G12D) allele in sorted hematopoietic populations from the AdCre/KRASG12D lung. The recombined Lox-G12D allele is clearly detected in CD45+CD11blowCD11c+ cells (population 2) at a similar level to the KRASWT (WT) allele. (B) Left, Giemsa staining of sorted CD45+CD11blowCD11c+ cells. This population is composed of small cells showing basophilic cytoplasm with relatively high nuclear/cytoplasmic (N/C) ratios, and larger macrophage-like cells. Scale bar, 10 μm. Right, confocal imaging of enriched CD11c+ cells stained for CD207 (green) and MAC2 (red). Reticular CD207 staining in cytoplasm and variable levels of membranous/cytoplasmic MAC2 staining detected in most cells. Scale bar, 10 μm. (C) CD207 expression in the AdCre/KRASG12D lung detected by IHC (top) and immunoblotting (bottom). Scale bars (top panels), 62.5 μm. DIC, differential interference contrast; SSC, side scatter.
CD207 expression in lung CD11c+cells driven by KRASG12D. (A) Polymerase chain reaction (PCR) detection of the recombined KRASG12D (Lox-G12D) allele in sorted hematopoietic populations from the AdCre/KRASG12D lung. The recombined Lox-G12D allele is clearly detected in CD45+CD11blowCD11c+ cells (population 2) at a similar level to the KRASWT (WT) allele. (B) Left, Giemsa staining of sorted CD45+CD11blowCD11c+ cells. This population is composed of small cells showing basophilic cytoplasm with relatively high nuclear/cytoplasmic (N/C) ratios, and larger macrophage-like cells. Scale bar, 10 μm. Right, confocal imaging of enriched CD11c+ cells stained for CD207 (green) and MAC2 (red). Reticular CD207 staining in cytoplasm and variable levels of membranous/cytoplasmic MAC2 staining detected in most cells. Scale bar, 10 μm. (C) CD207 expression in the AdCre/KRASG12D lung detected by IHC (top) and immunoblotting (bottom). Scale bars (top panels), 62.5 μm. DIC, differential interference contrast; SSC, side scatter.
Morphologically, the sorted CD11c population was composed of smaller round cells with a basophilic cytoplasm and larger macrophage-like cells (Figure 2B). Monocytes or neutrophils with typical morphologies were not identified. Because of the F4/80+ phenotype and histological findings that are consistent with LC/LCH characteristics (Figure 1A),2,12,14,28,29 we next performed CD207 immunostaining of enriched CD11c+ cells combined with a myeloid marker MAC2.30 We found that most cells showed reticular cytoplasmic CD207 staining with plasma membrane MAC2 (Figure 2B), suggesting that the CD11c+ cells are committed to LC-lineage differentiation. Consistently, CD207+ cell accumulation in the AdCre/KRASG12D lung was detected by IHC, and increased CD207 protein expression was confirmed by immunoblotting (Figure 2C).
In vitro development of KRASG12D-driven CD11c+F4/80+CD207+ cells
To investigate the proliferative potential of the CD11c+ cells, CD11c-enriched AdCre/KRASG12D lung cells were cultured with BrdU. However, BrdU uptake was almost negligible in the CD11chigh cells (Figure 3A), indicating that they were mitotically inactive. Interestingly, some BrdU incorporation was detected in CD11cdim cells (Figure 3A), but they failed to grow in long-term culture (data not shown). In contrast, when CD11chigh cells were removed by selective adhesion to plastic plates and the resulting “CD11c-depleted” nonadherent cells were cultured, floating cell clusters and endothelial (CD31+) networks developed along with fibroblasts and epithelial cells (Figure 3B). The majority of floating cells were CD45+CD11b+ small round cells that exhibited KRASG12D recombination (Figure 3B-C), demonstrating that the floating cells are myeloid cells promoted by KRASG12D expression. Even after 7 weeks of culture, they maintained proliferative potential, and expressed surface CD11c and F4/80 (Figure 3C). Notably, CD11chighF4/80high cells in this population partially expressed surface CD207, whereas CD11clowF4/80low cells did not (Figure 3C), suggesting that upregulation of CD11c and F4/80 could be associated with LC-lineage commitment.
In vitro development of KRASG12D-driven CD11c+F4/80+CD207+cells. (A) BrdU incorporation of freshly isolated AdCre/KRASG12D lung cells enriched for CD11c. Freshly harvested/enriched CD11c+ cells were cultured for 18 hours with BrdU, and analyzed by flow cytometry. Most CD11+ cells are BrdU−, though substantial BrdU uptake was detected in CD11clow cells (indicated in blue). (B) In vitro development of KRASG12D-expressing floating cell clusters in culture of AdCre/KRASG12D lung cells depleted for CD11c+ cells. Top, phase-contrast images of the culture at 2 weeks (left), 4 weeks (middle), and 7 weeks (right), showing floating cell cluster development during the culture. Original magnification ×200. Bottom, analyses of the culture at 7 weeks. Morphology of floating cells (left, Giemsa staining; scale bar, 10 μm), CD31+ endothelial network formation (middle, CD31 IHC; scale bar, 50 μm), and recombination of the KRASG12D allele in floating and adherent cells detected by PCR (left, with no-template/KRASWT controls) are indicated. The recombined KRASG12D allele (Lox-G12D) is clearly detected in the floating cells with much higher recombination rates than that in the adherent cells. (C) Flow cytometry analysis of the floating cells developing in CD11c-depleted AdCre/KRASG12D lung cell cultures at 7 weeks. Representative contour plots for cell surface CD45/CD11b (left), BrdU incorporation (18-hour labeling; second left) and surface CD11c/F4/80 (middle) are indicated. Cell surface CD207 expression in CD11chighF4/80high and CD11clowF4/80low populations (shown in the middle CD11c/F4/80 plot in green and blue, respectively) are indicated in histogram plots in the right (green for CD11chighF4/80high and blue for CD11clowF4/80low cells) with isotype (fluorescence minus one [FMO]) controls. (D) Representative phase-contrast (left, original magnification ×200) and CD207/MAC2 immunofluorescence (middle to right, by confocal laser-scanning microscopy) images of floating cluster cells replated at 7 weeks in culture. More than half of the cells show LC-like dendritic morphology, whereas some macrophage-like large round cells spreading on the culture plate are also seen (phase-contrast image in the left) at 48 hours following replating. LC-like cells are positive for CD207 at the plasma membrane with nuclear MAC2 staining (middle), but neither membrane CD207 nor nuclear MAC2 staining is detectable in macrophage-like cells (right). Scale bar, 10 μm (confocal images). MΦ, macrophage.
In vitro development of KRASG12D-driven CD11c+F4/80+CD207+cells. (A) BrdU incorporation of freshly isolated AdCre/KRASG12D lung cells enriched for CD11c. Freshly harvested/enriched CD11c+ cells were cultured for 18 hours with BrdU, and analyzed by flow cytometry. Most CD11+ cells are BrdU−, though substantial BrdU uptake was detected in CD11clow cells (indicated in blue). (B) In vitro development of KRASG12D-expressing floating cell clusters in culture of AdCre/KRASG12D lung cells depleted for CD11c+ cells. Top, phase-contrast images of the culture at 2 weeks (left), 4 weeks (middle), and 7 weeks (right), showing floating cell cluster development during the culture. Original magnification ×200. Bottom, analyses of the culture at 7 weeks. Morphology of floating cells (left, Giemsa staining; scale bar, 10 μm), CD31+ endothelial network formation (middle, CD31 IHC; scale bar, 50 μm), and recombination of the KRASG12D allele in floating and adherent cells detected by PCR (left, with no-template/KRASWT controls) are indicated. The recombined KRASG12D allele (Lox-G12D) is clearly detected in the floating cells with much higher recombination rates than that in the adherent cells. (C) Flow cytometry analysis of the floating cells developing in CD11c-depleted AdCre/KRASG12D lung cell cultures at 7 weeks. Representative contour plots for cell surface CD45/CD11b (left), BrdU incorporation (18-hour labeling; second left) and surface CD11c/F4/80 (middle) are indicated. Cell surface CD207 expression in CD11chighF4/80high and CD11clowF4/80low populations (shown in the middle CD11c/F4/80 plot in green and blue, respectively) are indicated in histogram plots in the right (green for CD11chighF4/80high and blue for CD11clowF4/80low cells) with isotype (fluorescence minus one [FMO]) controls. (D) Representative phase-contrast (left, original magnification ×200) and CD207/MAC2 immunofluorescence (middle to right, by confocal laser-scanning microscopy) images of floating cluster cells replated at 7 weeks in culture. More than half of the cells show LC-like dendritic morphology, whereas some macrophage-like large round cells spreading on the culture plate are also seen (phase-contrast image in the left) at 48 hours following replating. LC-like cells are positive for CD207 at the plasma membrane with nuclear MAC2 staining (middle), but neither membrane CD207 nor nuclear MAC2 staining is detectable in macrophage-like cells (right). Scale bar, 10 μm (confocal images). MΦ, macrophage.
When the floating cells were replated, they altered their morphology within 48 hours. Half of the cells showed LC-like dendritic morphology whereas one-third had a macrophage-like round/amoeboid morphology (Figure 3D). Plasma membrane CD207 expression and nuclear MAC2 localization were characteristic of LC-like cells and were not observed in the macrophage-like cells (Figure 3D). These in vitro data suggest the presence of proliferative precursors in the AdCre/KRASG12D lung that differentiate into CD11chighF4/80high LC-lineage cells.
Quantification of cells initiating CD11c+F4/80+ cell growth upon KRASG12D expression
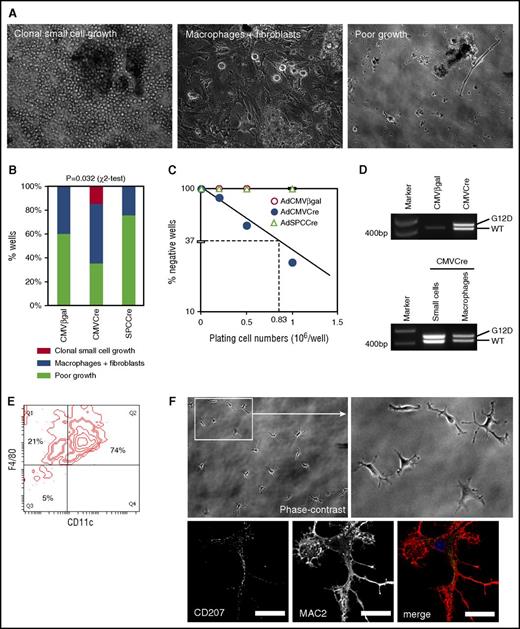
The high AdCMVCre virus dose required for CD11c+F4/80+ cell accumulation in the AdCre/KRASG12D lung suggested that cells initiating the pathology could be relatively rare. This prompted us to estimate the frequency of “myeloid pathology-initiating cells” (MPICs). To this end, we infected unrecombined KRASLSL-G12D lung cells in vitro with AdCMVCre, or control vectors expressing β-galactosidase (AdCMVβgal) or Cre under the surfactant protein C promoter (AdSPCCre).31 When 2 × 105 AdCMVCre-infected cells were cultured for 4 weeks, 15% of the culture developed cell clusters (Figure 4A-B) in a similar manner to the AdCre/KRASG12D lung cultures (Figure 3B). When the plating cell number was increased to 1 × 106, 75% of the culture produced cell clusters, by which the frequency of MPICs was estimated as 1 in 0.83 × 106 lung cells (95% confidence interval, 1 in 1.39 × 106 to 1 in 0.50 × 106) (Figure 4C). Floating cell clusters developing in these cultures showed clear KRASG12D recombination (Figure 4D). In contrast, cells infected with control vectors only developed mixed cultures with macrophage-like cells and fibroblasts (Figure 4A-B), indicating that KRASG12D expression was essential for the aberrant growth of small round cells forming cell clusters. Half of AdCMVCre-infected cultures plated at 2 × 105 cells per well also developed macrophage/fibroblast mixed cultures (Figure 4A-B), and floating macrophages collected from the cultures showed KRASG12D recombination (Figure 4D), suggesting that KRASG12D expression in lung macrophages might not be sufficient for initiating the development of cluster-forming cells.
Limiting dilution culture of lung cells induced to express KRASG12Din vitro. (A) Phase-contrast images of AdCMVCre-infected KRASLSL-G12D lung cell cultures (2 × 105 per well plating). Cultures developing floating cell clusters composed of small round cells (left), macrophage-like large round cells with fibroblasts (middle), and poorly growing cells (right), are indicated. Original magnification ×200. (B) Comparison between AdCMVCre-infected and control (AdCMVβgal or AdSPCCre-infected) cultures with regard to the development of floating round cell clusters and macrophage-like cells. A total of 20 wells plated at 2 × 105 per well (5 wells per mouse × 4 KRASLSL-G12D mice) for each infection were analyzed at 4 weeks in culture. Floating round cell cluster development was detected only in AdCMVCre-infected cultures (3 of 20 wells). Statistical significance of the difference was confirmed by χ2 test. (C) Limiting dilution analysis to determine the frequency of the cells initiating the development of floating round cell clusters. The data obtained from adenovirus-infected cultures plated at 2 × 105 per well (20 wells), 5 × 105 per well (12 wells), or 1 × 106 per well (8 wells), and floating cell cluster formation was determined at 4 weeks in culture. The frequency was estimated as 1 in 0.83 × 106 AdCMVCre-infected cells. (D) PCR detection of recombination of the KRASG12D allele in AdCMVCre or AdCMVβgal-infected cultures (top), and in small round cells forming floating cell clusters or macrophage-like large cells obtained from AdCMVCre-infected cultures (bottom). The recombined KRASG12D allele (Lox-G12D) was detected in both floating small cells and large macrophage-like cells developing in the AdCre-infected culture, at a similar level to the KRASWT (WT) allele. (E) Flow cytometry analysis of surface CD11c and F4/80 expression on small cells forming floating clusters at 7 weeks in the AdCMVCre-infected culture. A representative contour plot for CD11c and F4/80 is indicated. (F) Representative phase-contrast (top, original magnification ×200) and CD207/MAC2 immunofluorescence (bottom, by confocal laser scanning microscopy) images of replated cluster cells. Floating cells at 7 weeks in the AdCMVCre-infected culture were replated on coverslips, and imaged at 48 hours following replating. Scale bar, 20 μm (confocal images).
Limiting dilution culture of lung cells induced to express KRASG12Din vitro. (A) Phase-contrast images of AdCMVCre-infected KRASLSL-G12D lung cell cultures (2 × 105 per well plating). Cultures developing floating cell clusters composed of small round cells (left), macrophage-like large round cells with fibroblasts (middle), and poorly growing cells (right), are indicated. Original magnification ×200. (B) Comparison between AdCMVCre-infected and control (AdCMVβgal or AdSPCCre-infected) cultures with regard to the development of floating round cell clusters and macrophage-like cells. A total of 20 wells plated at 2 × 105 per well (5 wells per mouse × 4 KRASLSL-G12D mice) for each infection were analyzed at 4 weeks in culture. Floating round cell cluster development was detected only in AdCMVCre-infected cultures (3 of 20 wells). Statistical significance of the difference was confirmed by χ2 test. (C) Limiting dilution analysis to determine the frequency of the cells initiating the development of floating round cell clusters. The data obtained from adenovirus-infected cultures plated at 2 × 105 per well (20 wells), 5 × 105 per well (12 wells), or 1 × 106 per well (8 wells), and floating cell cluster formation was determined at 4 weeks in culture. The frequency was estimated as 1 in 0.83 × 106 AdCMVCre-infected cells. (D) PCR detection of recombination of the KRASG12D allele in AdCMVCre or AdCMVβgal-infected cultures (top), and in small round cells forming floating cell clusters or macrophage-like large cells obtained from AdCMVCre-infected cultures (bottom). The recombined KRASG12D allele (Lox-G12D) was detected in both floating small cells and large macrophage-like cells developing in the AdCre-infected culture, at a similar level to the KRASWT (WT) allele. (E) Flow cytometry analysis of surface CD11c and F4/80 expression on small cells forming floating clusters at 7 weeks in the AdCMVCre-infected culture. A representative contour plot for CD11c and F4/80 is indicated. (F) Representative phase-contrast (top, original magnification ×200) and CD207/MAC2 immunofluorescence (bottom, by confocal laser scanning microscopy) images of replated cluster cells. Floating cells at 7 weeks in the AdCMVCre-infected culture were replated on coverslips, and imaged at 48 hours following replating. Scale bar, 20 μm (confocal images).
Floating cell clusters were maintained in culture for 7 weeks, and their CD11c+F4/80+ phenotype was confirmed (Figure 4E). Morphological changes to LC-like cells were also observed upon replating (Figure 4F), but plasma membrane CD207 staining or MAC2 nuclear translocation was rarely detected in this condition. In the in vitro AdCMVCre infection culture, neither endothelial networks nor epithelial cells were developed. Such differences in the nonhematopoietic components observed following in vivo and in vitro KRASG12D induction might affect the CD207/MAC2 phenotype of the developed LC-like cells.
Lung-resident CD11bintCD11cdimGr1− cells initiate aberrant cell growth upon KRASG12D expression
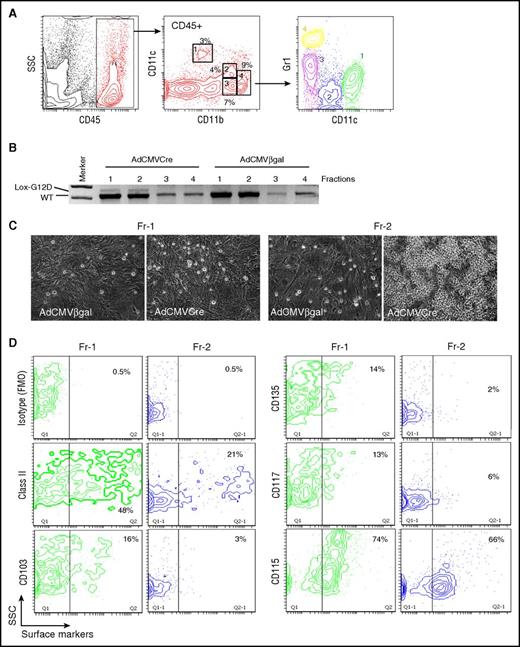
To prospectively evaluate MPIC potentials of lung myeloid populations, 4 myeloid subpopulations in uninduced KRASLSL-G12D lungs, including CD11blowCD11c+ (Fr-1), CD11bintCD11cdimGr1− (Fr-2), CD11bintCD11c−Gr1int (Fr-3), and CD11bhighCD11c−Gr1high (Fr-4) cells, were isolated and infected with AdCMVCre (Figure 5A-B). KRASG12D recombination was undetectable in Fr-3/4 cells that could not survive in this condition, but AdCMVCre-infected Fr-1/2 cells showed weak KRASG12D recombination (Figure 5B). When cultured without feeders, infected Fr-1/2 cells were viable for a couple of days, but eventually died within 10 days without showing any signs of active growth. However, AdCMVCre-infected Fr-2 cells proliferated to form round cell clusters on fibroblast feeders (Figure 5C), suggesting that lung-resident CD11bintCD11cdimGr1− cells were likely transformed into MPICs by KRASG12D expression. These data also suggest that the poor adenoviral infection efficiency into this population might be the major reason for the rarity of MPICs.
KRASG12D-induced aberrant growth of lung CD11bintCD11cdimGr1−cells. (A) Flow cytometry profiling of lung myeloid cells in noninduced KRASLSL-G12D mice. CD45+ gated lung hematopoietic cells contain CD11blowCD11c+ (Fr-1), CD11bintCD11cdimGr1− (Fr-2), CD11bintCD11c-Gr1int (Fr-3), and CD11bhighGr1high (Fr-4) myeloid populations. Gr1 expression profiles of the 4 myeloid populations are indicated in the bottom CD11c/Gr1 contour plot. (B) PCR detection of recombination of the KRASG12D allele in the sorted myeloid populations (Fr-1 to 4 from noninduced KRASLSL-G12D mice) infected with AdCMVCre or AdCMVβgal at MOI = 100 for 72 hours. (C) Representative phase-contrast images of AdCMVβgal or AdCMVCre-infected Fr-1/2 cells (sorted from noninduced KRASLSL-G12D mice) cultured on lung fibroblast feeders for 4 weeks. Original magnification ×200. (D) Flow cytometry analysis of noninduced Fr-1/2 cells for DC marker expression.
KRASG12D-induced aberrant growth of lung CD11bintCD11cdimGr1−cells. (A) Flow cytometry profiling of lung myeloid cells in noninduced KRASLSL-G12D mice. CD45+ gated lung hematopoietic cells contain CD11blowCD11c+ (Fr-1), CD11bintCD11cdimGr1− (Fr-2), CD11bintCD11c-Gr1int (Fr-3), and CD11bhighGr1high (Fr-4) myeloid populations. Gr1 expression profiles of the 4 myeloid populations are indicated in the bottom CD11c/Gr1 contour plot. (B) PCR detection of recombination of the KRASG12D allele in the sorted myeloid populations (Fr-1 to 4 from noninduced KRASLSL-G12D mice) infected with AdCMVCre or AdCMVβgal at MOI = 100 for 72 hours. (C) Representative phase-contrast images of AdCMVβgal or AdCMVCre-infected Fr-1/2 cells (sorted from noninduced KRASLSL-G12D mice) cultured on lung fibroblast feeders for 4 weeks. Original magnification ×200. (D) Flow cytometry analysis of noninduced Fr-1/2 cells for DC marker expression.
Although the CD11bintCD11cdimGr1− phenotype was consistent with resident/patrolling monocytes,32,33 CD11b+ DCs34 and interstitial macrophages35,36 could also show similar phenotypes. Therefore, we further analyzed Fr-2 cells for their expression of major histocompatibility complex class II and DC markers.37 They were found to be CD115+SSClow cells negative for DC markers CD103/CD117/CD135, but included class II+ subpopulations (Figure 5D), likely composed of professional antigen-presenting cells and/or transitional (inflammatory to resident) monocytes with class II upregulation.38
BRAFV600E expression in lung myeloid cells is not sufficient for inducing pulmonary LCH-like neoplasm
Because BRAF is frequently mutated in human pulmonary LCH,4,8,9,39-41 we investigated whether nasal delivery of AdCMVCre into the BRAF model also induced LCH-like neoplasms. AdCMVCre (3 × 107 pfu) was nasally delivered into the lung of BRAFLSL-V600E mice (referred to as AdCre/BRAFV600E mice hereafter), and lung tissues were histologically analyzed at 4 months following AdCMVCre infection. In contrast to AdCre/KRASG12D mice, well-demarcated papillary adenomas accompanied by myeloid cells in the microenvironment developed, especially in adenoma-rich areas (Figure 6A left) as we have previously reported.24 On the other hand, alveolar structures in adenoma-free areas were largely intact without developing DIP-like lesions (Figure 6A right).
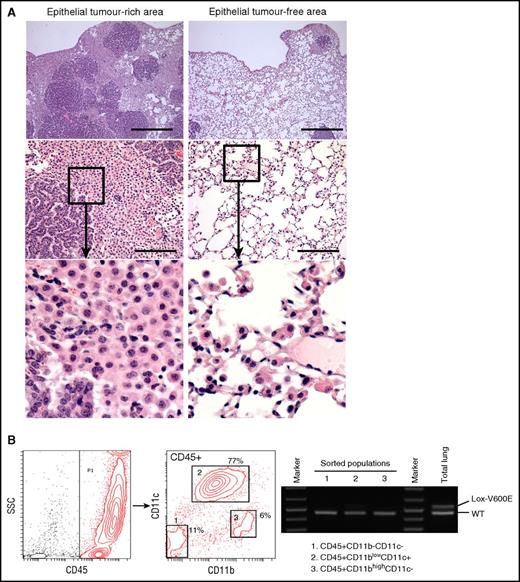
Characterization of the AdCre/BRAFV600Elung. (A) H&E staining of lung sections from AdCre/BRAFV600E mice. Low (top) and high (middle) magnification images focusing on epithelial tumor (papillary adenoma)-rich (left) and epithelial tumor-free (right) areas are indicated. Enlarged images for the boxed area in the middle panel are indicated at the bottom. Scale bars represent 0.5 mm (top) and 125 μm (middle). (B) PCR detection of the recombined BRAFV600E (Lox-V600E) allele in sorted hematopoietic populations from the AdCre/BRAFV600E lung. The recombined Lox-V600E allele was only faintly detected in CD45+CD11blow-intCD11c+ cells (population 2), and was much weaker than the BRAFWT (WT) allele. No recombination of the allele was detected in 2 other populations.
Characterization of the AdCre/BRAFV600Elung. (A) H&E staining of lung sections from AdCre/BRAFV600E mice. Low (top) and high (middle) magnification images focusing on epithelial tumor (papillary adenoma)-rich (left) and epithelial tumor-free (right) areas are indicated. Enlarged images for the boxed area in the middle panel are indicated at the bottom. Scale bars represent 0.5 mm (top) and 125 μm (middle). (B) PCR detection of the recombined BRAFV600E (Lox-V600E) allele in sorted hematopoietic populations from the AdCre/BRAFV600E lung. The recombined Lox-V600E allele was only faintly detected in CD45+CD11blow-intCD11c+ cells (population 2), and was much weaker than the BRAFWT (WT) allele. No recombination of the allele was detected in 2 other populations.
Although we previously reported reactive myeloid cell accumulation in the BRAFV600E model induced by spontaneous activation of CreER,24 the myeloid cells similarly accumulating in the AdCre/BRAFV600E lung were not formally proven to be nonneoplastic. To address this, we sorted hematopoietic subpopulations from the AdCre/BRAFV600E lung, and BRAF recombination was examined. Similarly to the AdCre/KRASG12D lung (Figure 2A), 3 distinct hematopoietic populations were identified, but the CD11c+ population in the AdCre/BRAFV600E lung showed only subtle recombination of the BRAFV600E allele (Figure 6B), confirming that CD11c+ cells in the BRAF model were largely nonneoplastic. These data demonstrate that BRAFV600E expression in lung-resident myeloid cells by nasal delivery of AdCMVCre is not sufficient for promoting myeloid neoplasms as seen in the AdCre/KRASG12D lung.
In vivo statin treatment inhibits accumulation of CD11c+ cells in the AdCre/KRASG12D lung
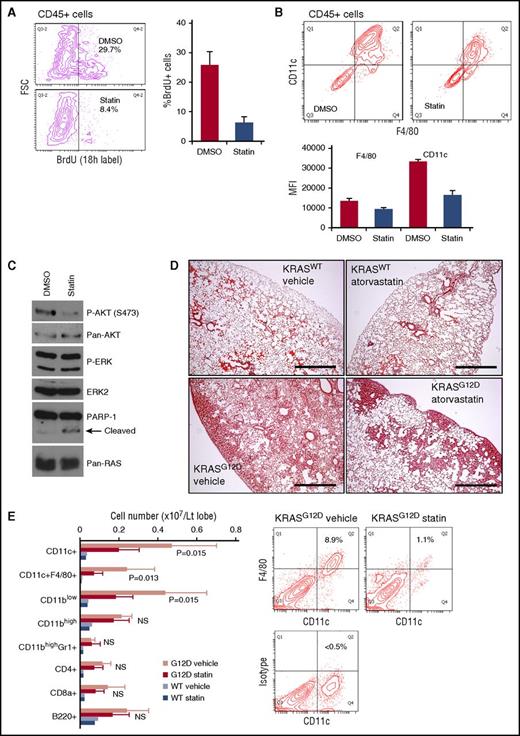
Isoprenylation of RAS proteins promotes their association with the plasma membrane where the proteins are activated.42 Consistently, genetic modifications inhibiting RAS prenylation have been reported to ameliorate KRAS-driven myeloproliferative disorders.43,44 Therefore, we sought to investigate whether chemical inhibition of protein prenylation suppresses myeloid neoplasm developing in the AdCre/KRASG12D mice. Because isoprenoid lipids synthesized through the mevalonate pathway are required for RAS prenylation,42 we used atorvastatin, an 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor that suppresses the mevalonate pathway upstream of isoprenoid lipid production. AdCre/KRASG12D lung cultures treated with 3.3 μM atorvastatin showed significant reduction of proliferating CD45+ cells compared with vehicle control cultures (Figure 7A). This antiproliferative effect was not associated with differentiation induction because CD11c and F4/80 expression was rather suppressed by atorvastatin (Figure 7B). Our biochemical analysis of CD11c-enriched AdCre/KRASG12D lung cells treated with atorvastatin showed decreased AKT, but not ERK, phosphorylation (Figure 7C), suggesting that inhibition of RAS prenylation inhibits RAS interaction with phosphatidylinositol 3-kinase (PI3K), as reported for marrow-derived macrophages.45 Of note, poly (ADP-ribose) polymerase 1 (PARP-1) cleavage was also induced by atorvastatin (Figure 7C), indicating that apoptosis of atorvastatin-treated CD11c+ cells might contribute to the reduced CD11c levels in atorvastatin cultures (Figure 7B).
Atorvastatin suppresses KRASG12D-induced lung myeloid pathology. (A) Effects of in vitro atorvastatin treatment on BrdU incorporation in CD45+ cells developing in AdCre/KRASG12D whole-lung cell cultures. Mixed-lineage cultures established from whole-lung cells during the first week were treated for 96 hours with dimethyl sulfoxide (DMSO) or 3.3 μM atorvastatin (statin) in serum-free media, and labeled with BrdU for 18 hours. BrdU incorporation in developed CD45+ cells was measured by flow cytometry. Representative contour plots are indicated on the left, and the percentage of BrdU+ in the CD45+ cells is summarized in the right bar graph (n = 3, mean + SD). (B) Effects of in vitro atorvastatin treatment on CD11c and F4/80 expression on CD45+ cells developing in AdCre/KRASG12D whole-lung cell cultures. Cells developed and treated as in panel A were analyzed by flow cytometry. Representative contour plots are indicated in the top, and mean fluorescence intensity (MFI) values of CD11c and F4/80 staining are summarized in the bottom bar graph (n = 3, mean + SD). (C) Immunoblot analysis of ERK/AKT phosphorylation and PARP-1 cleavage in AdCre/KRASG12D lung-derived CD11c+ cells treated with atorvastatin. Freshly harvested/enriched CD11c+ cells were cultured for 24 hours with 3.3 μM atorvastatin or DMSO in serum-free media for protein harvest. (D) H&E staining of lung sections from AdCre/KRASWT and AdCre KRASG12D mice treated with either vehicle or atorvastatin (10 mg/kg, daily for 5 weeks). Scale bar, 0.5 mm. (E) Lung hematopoietic-lineage cell numbers were quantitated in AdCre/KRASG12D mice treated with vehicle or atorvastatin (G12D vehicle/statin, n = 5 each), and control AdCre/KRASWT mice were similarly treated (WT vehicle/statin, n = 2 each). The bar graph summarizes each hematopoietic-lineage cell number per left lobe (mean + SD), and P values by Student t test between G12D vehicle/statin mice are indicated. Representative contour plots for CD11c-F4/80 staining of whole-lung cells obtained from G12D vehicle/statin mice are indicated on the right. FSC, forward scatter; NS, not significant; p-AKT, phosphorylated AKT; p-ERK, phosphorylated ERK.
Atorvastatin suppresses KRASG12D-induced lung myeloid pathology. (A) Effects of in vitro atorvastatin treatment on BrdU incorporation in CD45+ cells developing in AdCre/KRASG12D whole-lung cell cultures. Mixed-lineage cultures established from whole-lung cells during the first week were treated for 96 hours with dimethyl sulfoxide (DMSO) or 3.3 μM atorvastatin (statin) in serum-free media, and labeled with BrdU for 18 hours. BrdU incorporation in developed CD45+ cells was measured by flow cytometry. Representative contour plots are indicated on the left, and the percentage of BrdU+ in the CD45+ cells is summarized in the right bar graph (n = 3, mean + SD). (B) Effects of in vitro atorvastatin treatment on CD11c and F4/80 expression on CD45+ cells developing in AdCre/KRASG12D whole-lung cell cultures. Cells developed and treated as in panel A were analyzed by flow cytometry. Representative contour plots are indicated in the top, and mean fluorescence intensity (MFI) values of CD11c and F4/80 staining are summarized in the bottom bar graph (n = 3, mean + SD). (C) Immunoblot analysis of ERK/AKT phosphorylation and PARP-1 cleavage in AdCre/KRASG12D lung-derived CD11c+ cells treated with atorvastatin. Freshly harvested/enriched CD11c+ cells were cultured for 24 hours with 3.3 μM atorvastatin or DMSO in serum-free media for protein harvest. (D) H&E staining of lung sections from AdCre/KRASWT and AdCre KRASG12D mice treated with either vehicle or atorvastatin (10 mg/kg, daily for 5 weeks). Scale bar, 0.5 mm. (E) Lung hematopoietic-lineage cell numbers were quantitated in AdCre/KRASG12D mice treated with vehicle or atorvastatin (G12D vehicle/statin, n = 5 each), and control AdCre/KRASWT mice were similarly treated (WT vehicle/statin, n = 2 each). The bar graph summarizes each hematopoietic-lineage cell number per left lobe (mean + SD), and P values by Student t test between G12D vehicle/statin mice are indicated. Representative contour plots for CD11c-F4/80 staining of whole-lung cells obtained from G12D vehicle/statin mice are indicated on the right. FSC, forward scatter; NS, not significant; p-AKT, phosphorylated AKT; p-ERK, phosphorylated ERK.
The in vitro effects of atorvastatin prompted us to target the myeloid neoplasm in vivo. We thus treated AdCre/KRASG12D mice with atorvastatin for 5 weeks. In drug-treated animals, the DIP-like pathology was robustly improved, though some mass lesions, mainly composed of epithelial components, remained in the periphery of the lung (Figure 7D). Quantitative analysis using flow cytometry confirmed up to 70% reduction of CD11c+F4/80+ cells by atorvastatin, but no significant reduction was observed in other cells (Figure 7E). These data suggest the potential of statins as chemotherapeutic agents against RAS-mutated pulmonary LCH.
Discussion
Due to its distinct clinical characteristics, pulmonary LCH requires management/therapeutic strategies different from multisystem LCH.46 To better understand the pathogenesis of pulmonary LCH, and broaden therapeutic options for patients struggling with advanced diseases, development of animal models recapitulating the human disease is essential. In the present study, we found that adenoviral induction of KRASG12D expression in lung-resident myeloid cells promotes pulmonary LCH-like neoplasm in mice, and that the pathology can be ameliorated by in vivo administration of atorvastatin, a cholesterol-lowering drug widely used in the clinic. These data shed light on the potential cellular origin and genetic/molecular pathogenesis of pulmonary LCH, and provide a rationale to explore the therapeutic potential of statins against subsets of pulmonary LCH.
As far as we know, this is the first demonstration that nasal delivery of AdCMVCre to the KRASLSL-G12D mouse lung can induce hematopoietic neoplasms. Our data show that nasal delivery of AdCMVCre at relatively high doses is required for inducing myeloid neoplasms, likely due to relatively few cells in the lung initiating neoplastic myeloid growth (Figure 4). However, the lung pathology induced by high-dose AdCMVCre is complex, with hematopoietic phenotypes coexisting with alveolar and bronchiolar neoplasms. These factors may have collectively contributed to the fact that KRASG12D-induced neoplastic transformation of lung-resident myeloid cells in this model has not been previously appreciated.
To our surprise, the majority of pathogenic CD11chigh cells in the AdCre/KRASG12D lung were found to be mitotically inactive (Figure 3A), though this is consistent with a previous study showing nonproliferative lesional cells in human pulmonary LCH.47 Instead, proliferating CD45+ cells expressing variable levels of CD11c developed in culture, suggesting that proliferating precursors for CD11chigh cells can exist in the AdCre/KRASG12D lung. Proliferating CD45+ cells form cell clusters (Figure 3B) in a similar way to immature LCs developing from human CD34+ hematopoietic stem/progenitor cells (HSPCs) cultured with transforming growth factor β1 (TGFβ1).48 The CD11c-depleted culture was composed of not only hematopoietic, but also epithelial, fibroblastic, and endothelial cells (Figure 3B) and our preliminary analysis detected TGFβ1 in the culture media (T.K. and C.P., unpublished data). Further investigations to identify the cellular source(s) for the secreted TGFβ1 are currently under way.
Although our data suggest that CD11bintCD11cdimGr1− monocytes are the major cellular targets of KRASG12D-induced transformation in our model, the in vitro infection efficiency of AdCMVCre into this population was very low (Figure 5B). This could be due to the low-level expression of αv integrins that mediate adenovirus internalization.49 Granulocyte-macrophage colony-stimulating factor (GMCSF) stimulation of human monocytes reportedly induces αv integrin expression, leading to improved adenoviral gene delivery,49 whereas alveolar epithelial cells are the major GMCSF-producing cells in mouse lungs.50 Alveolar epithelium-derived GMCSF may induce αv integrin expression in vivo in lung-resident monocytes, making them more susceptible to adenoviral infection.
BRAF is frequently mutated in human pulmonary LCH (24%-63%)4,8,9,39-41 with much higher prevalence than KRAS (8%),9 though NRAS mutations could be more common than KRAS.8 But unexpectedly, a pulmonary LCH-like neoplasm was not observed in our AdCre/BRAF model (Figure 6). This may be explained by different cell populations initiating the BRAF-induced pathology. Our data suggest CD11bintCD11cdimGr1− monocytes as the major cell type initiating the KRASG12D-driven pathology, whereas common DC progenitors (CDPs) and Langerin+ DCs have been proposed to drive BRAFV600E-induced neoplasms.13 Nasally delivered AdCMVCre may not effectively infect lung-resident CDPs or Langerin+ DCs, resulting in the failure to develop LCH-like neoplasms. BRAFV600E-induced MEK/ERK activation may be sufficient for transforming CDPs and Langerin+ DCs, but KRASG12D-induced PI3K activation may be needed to transform CD11bintCD11cdimGr1− monocytes, as suggested by our atorvastatin data (Figure 7C).
Using our model as a platform for preclinical evaluation, we identified antitumor effects of atorvastatin against KRASG12D-driven pulmonary LCH-like neoplasm (Figure 7). Antigrowth effects of statins on tumor cells are thought to be attributable, at least in part, to inhibition of RAS family small GTPases.51 Our biochemical data (Figure 7C) suggest that atorvastatin inhibits prenyl-dependent association between KRAS and PI3K as reported in simvastatin-treated human peripheral blood mononuclear cells.45 Intriguingly, PIK3CA/BRAF comutations have been identified in Erdheim-Chester disease, a histiocytic disorder closely related to LCH,52 suggesting that cooperative activation of the MEK/ERK and PI3K pathways could contribute to the pathogenesis of histiocytic neoplasms. Although cancer-associated PIK3CA mutations are rare in LCH,53 we previously reported contribution of autocrine PI3K activation to the aberrant growth of BRAFV600E-expressing mouse HSPCs.25 Autocrine PI3K activation may also contribute to the pathogenesis of human multisystem LCH, in which marrow CD34+ HSPCs reportedly harbor BRAF mutations.13 In this regard, PI3K-targeted therapeutics may be beneficial for treating refractory LCH, not restricted to RAS-mutated pulmonary diseases but including BRAF-mutated multisystem diseases.
We exploited an autochthonous GEM model to investigate how KRASG12D expression in the lung affects lung-resident myeloid cells in a spatiotemporally controlled manner, leading to a unique opportunity to model pulmonary LCH. However, the clinical relevance of our preclinical study has yet to be fully explored in the clinical setting, though recent discoveries of RAS mutations in human pulmonary LCH are consistent with our findings. Future investigations of pulmonary LCH cases with regard to correlation between statin use and clinical outcomes/RAS mutation status would provide valuable information. Notably, a recent report of a phase 2 clinical trial of pan-AKT inhibitor afuresertib for LCH patients, including 7 evaluable cases with pulmonary involvement, demonstrated that AKT inhibition exerts limited efficacy against pulmonary LCH patients.54 Because AKT inhibition induces feedback activation of PI3K,55 which could signal through AKT-independent downstream pathways,56 AKT may not be an optimal target to suppress the PI3K pathway. Direct inhibition of PI3K isoform(s), rather than AKT inhibitors, would be warranted in future clinical trials for pulmonary LCH patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to the Department of Biomedical Services and the Advanced Imaging Facilities within Core Biotechnology Services at Leicester. The authors are also grateful to Karen Brown and Jennifer Higgins for assistance with cell-sorting experiments.
This work was supported by a Cancer Research UK programme grant (Ref. C1362/A13083). C.P. was also supported by a Royal Society-Wolfson Research Merit Award.
Authorship
Contribution: T.K. conceived and designed the entire study, performed experiments, analyzed data, and prepared figures; S.G. contributed to animal management and tissue preparation; and T.K. and C.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tamihiro Kamata, Department of Cancer Studies, University of Leicester, Lancaster Rd, Leicester LE1 9HN, United Kingdom; e-mail: tk83@le.ac.uk.

![Figure 3. In vitro development of KRASG12D-driven CD11c+ F4/80+ CD207+ cells. (A) BrdU incorporation of freshly isolated AdCre/KRASG12D lung cells enriched for CD11c. Freshly harvested/enriched CD11c+ cells were cultured for 18 hours with BrdU, and analyzed by flow cytometry. Most CD11+ cells are BrdU−, though substantial BrdU uptake was detected in CD11clow cells (indicated in blue). (B) In vitro development of KRASG12D-expressing floating cell clusters in culture of AdCre/KRASG12D lung cells depleted for CD11c+ cells. Top, phase-contrast images of the culture at 2 weeks (left), 4 weeks (middle), and 7 weeks (right), showing floating cell cluster development during the culture. Original magnification ×200. Bottom, analyses of the culture at 7 weeks. Morphology of floating cells (left, Giemsa staining; scale bar, 10 μm), CD31+ endothelial network formation (middle, CD31 IHC; scale bar, 50 μm), and recombination of the KRASG12D allele in floating and adherent cells detected by PCR (left, with no-template/KRASWT controls) are indicated. The recombined KRASG12D allele (Lox-G12D) is clearly detected in the floating cells with much higher recombination rates than that in the adherent cells. (C) Flow cytometry analysis of the floating cells developing in CD11c-depleted AdCre/KRASG12D lung cell cultures at 7 weeks. Representative contour plots for cell surface CD45/CD11b (left), BrdU incorporation (18-hour labeling; second left) and surface CD11c/F4/80 (middle) are indicated. Cell surface CD207 expression in CD11chighF4/80high and CD11clowF4/80low populations (shown in the middle CD11c/F4/80 plot in green and blue, respectively) are indicated in histogram plots in the right (green for CD11chighF4/80high and blue for CD11clowF4/80low cells) with isotype (fluorescence minus one [FMO]) controls. (D) Representative phase-contrast (left, original magnification ×200) and CD207/MAC2 immunofluorescence (middle to right, by confocal laser-scanning microscopy) images of floating cluster cells replated at 7 weeks in culture. More than half of the cells show LC-like dendritic morphology, whereas some macrophage-like large round cells spreading on the culture plate are also seen (phase-contrast image in the left) at 48 hours following replating. LC-like cells are positive for CD207 at the plasma membrane with nuclear MAC2 staining (middle), but neither membrane CD207 nor nuclear MAC2 staining is detectable in macrophage-like cells (right). Scale bar, 10 μm (confocal images). MΦ, macrophage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/4/10.1182_blood-2017-02-770149/4/m_blood770149f3.jpeg?Expires=1764979537&Signature=eKfmeJSArbsiyXiQ3IN7QOTb7tKNCL1MmApqxUQ9WKw22k0bUQAiEkvGDSLXsZ8zm2PAvR5Gvq44Ad8lSQ35YnkRgx73sORj4xZoTzFPqOjUPZaCOB5sQPuKduPxp3P7TUoTbtk1DNB2PH1Q0V50TL3htgCeB~zXPC9TpJ6O-UAWWdAIO9PP9rfRQnabzNraH1Rcn-3cBhmwx6ZZDKNGS4CRxnAQNSOjvZ39lTuRciKN9cNweIVYk8ikOSczlGdAwnusGF-0TwIpSH4yXViRfXrss5JT-bCjbZTeLb7o3UIJNOBT6BnYg~MAa4alSJuOyenU6nD5dISFtBNmvMzIDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal