Key Points
High anti-ADAMTS13 antibody and low ADAMTS13 antigen levels adversely affect outcome in immune-mediated TTP with greater mortality seen.
A raised troponin at presentation confers a sixfold increase and reduced GCS a nine-fold increase in mortality in acute TTP.
Abstract
Immune-mediated thrombotic thrombocytopenic purpura (TTP) is a life-threatening disorder caused by antibodies against ADAMTS13. From the United Kingdom TTP registry, we undertook a prospective study investigating the impact of the presenting anti-ADAMTS13 IgG antibody and ADAMTS13 antigen on mortality. A total of 312 episodes involving 292 patients over 87 months were included; 68% were female, median age 46 (range, 11-88 years), and median presenting ADAMTS13 of <5% (range, <5%-18%). The mortality rate was 10.3% (n = 32); 68% of patients had a raised troponin at presentation conferring a sixfold increase in mortality compared with those with normal troponin levels (12.1% vs 2.0%, P = .04). Twenty-four percent had a reduced Glasgow Coma Score (GCS) at presentation with a ninefold increase in mortality (20% vs 2.2% for normal GCS at presentation, P < .0001). Mortality increased with higher anti-ADAMTS13 antibody levels and lower ADAMTS13 antigen levels. Those with antibody levels in the upper quartile (antibody >77%) had a mortality of 16.9% compared with 5.0% for the lowest quartile (antibody <20%) (P = .004). Those with an antigen level in the lowest quartile (antigen <1.5%) had a mortality of 18% compared with 3.8% for the highest quartile (antigen >11%) (P = .005). The synergistic effect of anti-ADAMTS13 IgG antibody in the upper quartile and ADAMTS13 antigen in the lowest quartile had the highest mortality of 27.3%. We conclude that both anti-ADAMTS13 IgG antibody and ADAMTS13 antigen levels correlate with outcome in TTP with increased cardiac and neurological involvement and increased mortality.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare, life threatening disorder with a reported incidence of between 4 and 13 cases per million per year,1-4 at least 3 times more common in women,2,5 and with an untreated mortality of up to 90%.3,6 Severe deficiency of the metalloprotease ADAMTS13 results in the abnormal presence of circulating ultra large von Willebrand factor (VWF) multimers. Decreased ADAMTS13 can occur as a result of an inherited deficiency or an acquired immune-mediated deficiency; as such, 2 categories exist: congenital TTP and immune-mediated TTP. In the latter, ADAMTS13 activity levels are severely reduced by antibody-mediated activity against ADAMTS13.
There remains a limited ability to prognosticate in TTP, a significant problem because mortality remains relatively unchanged at 10% to 20% with treatment.7,8 A major challenge is to reliably identify patients with high-risk disease at presentation who require and might benefit from early intensification of therapy. This has led to an investigation of factors that may have an impact on outcome. A number of studies have found that lower ADAMTS13 activity at presentation is a poor prognostic indicator.7-9 Similarly, increasing age has been found to be an independent risk factor for poor prognosis in multiple studies.10-12 There is a growing belief that ethnicity is important, with some suggestion that African American patients are at greater risk of TTP relapse,8 whereas white patients may have a worse prognosis.12 A raised serum lactate dehydrogenase level,10 raised cardiac troponin,13,14 and severe neurological involvement10,12 have all been identified as further prognostic markers.
One potential area of interest is the immunological basis behind the TTP disease process, specifically the role of the anti-ADAMTS13 antibody and corresponding ADAMTS13 antigen. In immune-mediated TTP, antibody-mediated activity against ADAMTS13 is identified in almost all patients,15,16 with antibodies usually of the immunoglobulin G (IgG) isotype,15-17 although IgM and IgA antibodies have also been documented.18,19 We have previously shown that increased anti-ADAMTS13 IgG antibody levels are a risk factor for poor outcome when combined with elevated cardiac troponin levels.13 However, there is little evidence for the significance of the antibody level in isolation, other than that TTP remission is associated with reducing anti-ADAMTS13 IgG antibody levels.20 The role of ADAMTS13 antigen levels in TTP is even less well characterized; however, emerging evidence suggests depletion of the ADAMTS13 antigen may be the dominant pathologic mechanism21 and its levels a significant prognostic indicator.22
Using the UK TTP registry, we have investigated whether the presenting levels of anti-ADAMTS13 IgG antibody and ADAMTS13 antigen had any effect on the mortality of patients presenting with acute TTP. As other studies have suggested, with a raised cardiac troponin level13,14 and neurological involvement10,12 as poor prognostic markers, these were also included in our investigation of risk factors for mortality in acute TTP.
Methods
Since 2009, the UK TTP Registry has been collecting information and samples on acute presentations of TTP from all treating sites across the country. Data collected include demographic information, ADAMTS13 activity, laboratory and organ damage markers at acute presentation, and subsequent follow-up (Medical Research Ethics Committee (MREC) number: 08/H0810/54).
Only immune-mediated TTP episodes have been considered with congenital TTP; all other thrombotic microangiopathies were excluded. Anti-ADAMTS13 IgG antibody and ADAMTS13 antigen levels were compared in patients who survived with those who died. Neurological involvement has been captured using a reduced Glasgow Coma Scale (GCS),23 whereas raised cardiac troponin was defined as any value above the upper limit of normal. Because patients presented to numerous hospitals, the upper limit of normal differed between sites. The upper limit of normal for both troponin I and troponin T were determined by the local laboratory range. All hospital laboratories participated in appropriate external quality assessment for validation of their troponin assay.
All blood samples were from the initial presentation and taken before the first plasma exchange. Acute TTP was defined as ADAMTS13 protease activity <10% (FRETS VWF-73 assay; normal range, 64% to 134%) or between 10% and 20% with a detectable IgG anti-ADAMTS13 IgG antibody present (IgG antibody normal range, <6%). The latter criteria were included to account for patients who received treatment with an infusion of fresh frozen plasma before being transferred to a center experienced in treating TTP. ADAMTS13 activity was measured using the FRETS VWF73 method24 and a published enzyme-linked immunosorbent assay technique18,25 for anti-ADAMTS13 IgG antibody quantification. Percentage values for the anti-ADAMTS13 IgG antibody levels were used as the upper limit of normal for the anti-ADAMTS13 IgG was 6% and this was calculated as the 95th percentile of 49 normal, healthy controls. Antibodies in the upper and lower quartiles were confirmed as inhibitory using a 50:50 mixing study with pooled normal plasma and activity measured by the FRETS VWF73 methodology as described previously. A strong inhibitor was defined as persisting ADAMTS13 activity <10% after attempts at correction with 50:50 mixing studies.
ADAMTS13 antigen levels were quantified using a newly developed in-house developed enzyme-linked immunosorbent assay. Plates were covered with 3H9 mouse monoclonal anti-human ADAMTS13 antibody incubated overnight. Following washing, these were blocked with phosphate-buffered saline (PBS) and 3% dried milk powder for 1 hour. A standard curve was created with dilutions of pooled normal plasma. All standards, tests, and controls were diluted 1:100 in PBS. Samples, standards, and controls were then added to the duplicate wells on the microplate and incubated for 1 hour at 37°C. After washing, 2 detection biotinylated-conjugated mouse monoclonal anti-human ADAMTS13 antibodies were prepared in PBS, “17G2” biotinylated at 1.5 µg/mL, and “19H4” biotinylated at 1.5 µg/mL and added to each well and incubated at room temperature for 1 hour. After washing, streptavidin-horseradish peroxidase, 1:10 000 in PBS was added to each well to bind biotin and incubated at room temperature for 1 hour before a final wash and the addition of 160 µL of fresh substrate to each well (15 mg o-phenylenediamine dihydrochloride, Sigma-Aldrich + 24 mL citrate phosphate buffer + 12 µL 6% hydrogen peroxide). The reaction was stopped with 4M sulfuric acid before plates were read using a Dynex spectrophotometer at 490 nm. Using 100 normal controls, a normal range of 74% to 134% was determined with a sensitivity of 0.5% based on the lower limit of detection. The intra-assay and inter-assay coefficients of variability were 3.57% and 5.04%, respectively.
All patients were included in the statistical analysis with the χ2 test, Student t test, Mann-Whitney U test, or Kruskal-Wallis test used as appropriate. GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA) was used for all statistical analyses.
Results
From 550 acute TTP episodes, 312 with complete data and samples were identified from the UK TTP Registry involving 292 patients between 2009 and 2016. Twenty-four centers treated 88% of all identified episodes; 68% of all patients were female, with a median age of 46 years at the time of the acute event (range, 11-88 years). Fifty-three percent of episodes involved white patients, with a further 22% of patients being of Afro-Caribbean descent. The overall mortality rate was 10.3% (n = 32), with a median time to death from first presentation of 4 days (range, 1-39 days). For the whole cohort (n = 312), the median ADAMTS13 activity at presentation was <5% (range, <5%-18%). Fifteen cases had presenting ADAMTS13 levels between 10% and 20%, but associated with raised anti-ADAMTS13 IgG antibody levels. Of these, 3 were relapsed disease with previously documented ADAMTS 13 activity <10%. In the remaining cases, repeat samples 24 to 48 hours later confirmed ADAMTS13 levels <10%. For the whole cohort, the median anti-ADAMTS13 IgG antibody level was 40% (range, 1%-189%) and the median ADAMTS13 antigen level was 4% (range, 0.5%-146%).
Demographic features of patients who died and survived are summarized in Table 1. There were no significant differences in the baseline demographic characteristics between patients who died and those who survived. However, the median anti-ADAMTS13 IgG antibody level at presentation was significantly higher in patients who died compared with survivors (died median IgG, 66% [4%-164%] vs survived 39% [1% to 189%], P = .004) (Figure 1). ADAMTS13 antigen level was lower in patients who died compared with those who survived (died median ADAMTS13 antigen, 1.6% (0.5-21.5) vs survived 4.2% (0.5%-146%), P = .0007) (Figure 2).
Demographic and ADAMTS13 activity data in patients who died or survived
| . | TTP episodes (n = 312) . | ||
|---|---|---|---|
| . | Survived (n = 280) . | Died (n = 32) . | P value . |
| Age (range) | 46 (11-85) | 51 (22-88) | .11* |
| Female (%) | 192 (69) | 20 (63) | .55† |
| Ethnicity (%) | |||
| Afro-Caribbean | 61 (22) | 7 (22) | >.99† |
| Asian | 18 (6) | 2 (6) | >.99† |
| White | 149 (53) | 16 (50) | .85† |
| Mixed/other | 16 (6) | 4 (13) | .14† |
| Not stated | 36 (13) | 3 (9) | .78† |
| Median ADAMTS13 activity (range), % | <5 (<5-18) | <5 (<5-18) | .92* |
| Median anti-ADAMTS13 IgG antibody level (range), % | 39 (1-189) | 66 (4-164) | .004* |
| Median ADAMTS13 antigen level (range) | 4.2 (0.5-146) | 1.6 (0.5-21.5) | .0007* |
| Relapsed TTP (%) | 37 (13.2) | 2 (6.3) | .39† |
| . | TTP episodes (n = 312) . | ||
|---|---|---|---|
| . | Survived (n = 280) . | Died (n = 32) . | P value . |
| Age (range) | 46 (11-85) | 51 (22-88) | .11* |
| Female (%) | 192 (69) | 20 (63) | .55† |
| Ethnicity (%) | |||
| Afro-Caribbean | 61 (22) | 7 (22) | >.99† |
| Asian | 18 (6) | 2 (6) | >.99† |
| White | 149 (53) | 16 (50) | .85† |
| Mixed/other | 16 (6) | 4 (13) | .14† |
| Not stated | 36 (13) | 3 (9) | .78† |
| Median ADAMTS13 activity (range), % | <5 (<5-18) | <5 (<5-18) | .92* |
| Median anti-ADAMTS13 IgG antibody level (range), % | 39 (1-189) | 66 (4-164) | .004* |
| Median ADAMTS13 antigen level (range) | 4.2 (0.5-146) | 1.6 (0.5-21.5) | .0007* |
| Relapsed TTP (%) | 37 (13.2) | 2 (6.3) | .39† |
Mann-Whitney U test.
χ2 test.
Anti-ADAMTS13 antibody and ADAMTS13 antigen results for individual patients, comparing those who died and survived. (A) Anti-ADAMTS13 antibody results; (B) ADAMTS13 antigen results. The overlying line shows the median, upper, and lower quartiles for each group. Median anti-ADAMTS13 IgG antibody levels were higher and median ADAMTS13 antigen levels lower in those who died than those who survived.
Anti-ADAMTS13 antibody and ADAMTS13 antigen results for individual patients, comparing those who died and survived. (A) Anti-ADAMTS13 antibody results; (B) ADAMTS13 antigen results. The overlying line shows the median, upper, and lower quartiles for each group. Median anti-ADAMTS13 IgG antibody levels were higher and median ADAMTS13 antigen levels lower in those who died than those who survived.
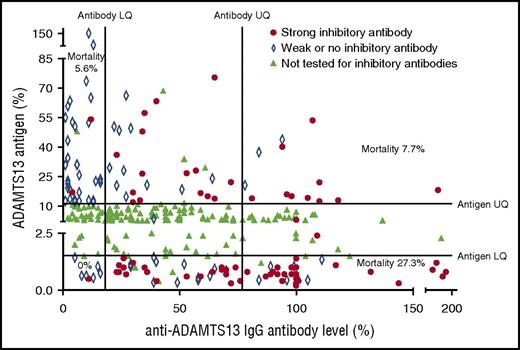
The highest rate of mortality was seen in patients with a high anti-ADAMTS13 antibody level and low ADAMTS13 antigen level. A total of 72.7% of these patients was found to have strong inhibitory antibodies on 50:50 mixing studies. The lowest rate of mortality was seen in patients with an antibody level in the lowest quartile (LQ); only 7% of these patients were found to have strong inhibitory antibodies. ADAMTS13 antigen levels plotted against anti-ADAMTS13 IgG antibody levels. Results in the upper or lower quartile for either factor were tested to confirm the presence of strong inhibitory antibodies (defined as those in which ADAMTS13 activity remained <10% after 50:50 mixing studies). These were most frequently seen in those with a low ADAMTS13 antigen level and a high anti-ADAMTS13 IgG antibody level. The largest cluster can be seen in those samples that fell into both of these groups with a corresponding increased level of mortality.
The highest rate of mortality was seen in patients with a high anti-ADAMTS13 antibody level and low ADAMTS13 antigen level. A total of 72.7% of these patients was found to have strong inhibitory antibodies on 50:50 mixing studies. The lowest rate of mortality was seen in patients with an antibody level in the lowest quartile (LQ); only 7% of these patients were found to have strong inhibitory antibodies. ADAMTS13 antigen levels plotted against anti-ADAMTS13 IgG antibody levels. Results in the upper or lower quartile for either factor were tested to confirm the presence of strong inhibitory antibodies (defined as those in which ADAMTS13 activity remained <10% after 50:50 mixing studies). These were most frequently seen in those with a low ADAMTS13 antigen level and a high anti-ADAMTS13 IgG antibody level. The largest cluster can be seen in those samples that fell into both of these groups with a corresponding increased level of mortality.
Factors affecting mortality
A troponin measurement above the upper limit of normal at presentation was seen in 68% of all patients at presentation. Patients with a cardiac troponin measurement above the local normal range had a sixfold increase in mortality of 12.1%, compared with those with troponin levels within the normal range where mortality was 2.0% (P = .04). Reduced GCS, defined as a GCS score of 14 or below, was documented in 28% of patients at presentation and associated with a ninefold increase in mortality rate (20% vs 2.2% for normal GCS at presentation, P < .0001). There was no synergistic effect on the mortality rate of a combined decreased GCS and an elevated cardiac troponin compared with the mortality for a single abnormal prognostic factor (mortality with both reduced GCS and raised troponin = 23.7%, P = .45).
Relapsed TTP
There were 39 cases, with relapsed TTP in 36 patients. Nineteen patients had their initial episode outside of the time of the registry, so their first documented event as part of the UK registry was with an acute TTP relapse. The remaining 17 patients had both their initial presentation and a relapse within the period of the registry and included in this cohort. Three patients had 2 relapses within the period of the registry.
There were notable differences in results between patients who presented with de novo TTP and those who presented with relapsed disease (Table 2). Anti-ADAMTS13 IgG antibody results were higher in patients who presented with de novo TTP (first episode, 48% [7%-189%] vs relapse 23% [1%-118%], P < .0001), and ADAMTS13 antigen levels were lower (first episode, 3.3% [0.5%-146.2%] vs relapse 9% [0.5%-68.9%], P = .0003). There was, however, no significant difference in mortality when comparing relapsed and de novo TTP (de novo mortality, 11%; relapsed mortality, 5.1%; P = .26). The frequency of raised troponin levels and neurological involvement are reduced in those with relapsed TTP (Table 2).
Comparing results for de novo and relapsed TTP patients
| . | De novo TTP (n = 273), % . | Relapsed TTP (n = 39), % . | P value . |
|---|---|---|---|
| Median ADAMTS13 activity (range) | <5 (<5-18) | <5 (<5-17) | .30 |
| Median anti-ADAMTS13 IgG antibody level (range) | 48 (7-189) | 23 (1-118) | <.0001 |
| Median ADAMTS13 antigen level (range) | 3.4 (0.5-146.2) | 9 (0.5-68.9) | .0004 |
| % GCS <15 | 26 | 12.8 | .07 |
| % Raised troponin | 74 | 36 | .03 |
| % Mortality | 11 | 5 | .26 |
| . | De novo TTP (n = 273), % . | Relapsed TTP (n = 39), % . | P value . |
|---|---|---|---|
| Median ADAMTS13 activity (range) | <5 (<5-18) | <5 (<5-17) | .30 |
| Median anti-ADAMTS13 IgG antibody level (range) | 48 (7-189) | 23 (1-118) | <.0001 |
| Median ADAMTS13 antigen level (range) | 3.4 (0.5-146.2) | 9 (0.5-68.9) | .0004 |
| % GCS <15 | 26 | 12.8 | .07 |
| % Raised troponin | 74 | 36 | .03 |
| % Mortality | 11 | 5 | .26 |
Anti ADAMTS13 IgG antibody level at presentation and TTP mortality
An abnormally elevated anti-ADAMTS13 IgG antibody level was seen in 90% of patients. Of the 10% with a low antibody level (n = 31), one-third were relapsed TTP (n = 10) with previously documented inhibitory antibody activity and a further 26% of this cohort (n = 8) would subsequently have an elevated antibody level measured later on during their TTP episode. Thirteen patients (4.2% of total study population) therefore did not display elevated anti-ADAMTS13 IgG antibody levels but maintained normal ADAMTS13 levels after rituximab treatment in keeping with a diagnosis of immune-mediated TTP rather than the congenital form.
Patients with an anti-ADAMTS13 IgG antibody level in the upper quartile had a mortality rate more than 3 times higher than patients with an anti-ADAMTS13 IgG antibody level in the lowest quartile (mortality IgG <20% = 5.0%, mortality IgG >77% = 16.9%, P = .02). Those in the second and third quartiles had a mortality rate of 6.5% and 12.8%, respectively. When comparing the upper and lower quartile, those in the upper quartile were also more likely to have a raised troponin (IgG <20% = 44% vs IgG >77% = 87%, P < .0001), a reduced GCS (IgG <20% = 19% vs IgG >77% = 41%, P = .035) and required a longer period of plasma exchange needed to achieve a normal platelet count (IgG <20% 10 sessions, IgG >77% 20 sessions, P = .006).
ADAMTS13 antigen level at presentation and TTP mortality
Ninety-nine percent of patients had an ADAMTS13 antigen level below the normal range at presentation. Mortality increased with decreasing antigen levels. Those with an antigen level in the lowest quartile (antigen <1.5%) had a mortality of 18.4% compared with 3.8% for those in the highest quartile (antigen >10%) (P = .005). Those in the second (antigen, 1.5% to 3.7%) and third quartiles (antigen, 3.9% to 10.8%) had a mortality rate of 10.4% and 8.9%, respectively. There was no statistically significant difference in the frequency of abnormal cardiac troponin measurements or abnormal GCS when comparing the upper and lower quartiles. There was no difference in the amount of plasma exchange required to achieve a normal platelet count when comparing those in the highest and lowest quartiles (antigen <1.5% 15 sessions, antigen >11% 13 sessions, P = .4). Four patients had normal or near-normal ADAMTS13 antigen levels (>70%). All were de novo cases with ADAMTS13 activity levels <10% and raised anti-ADAMTS13 IgG antibody levels. Of these, only 1 patient had a strong inhibitor detected by 50:50 mixing studies. There was no evidence of cardiological or neurological involvement in any of these cases. All 4 patients survived.
ADAMTS13 antigen levels further discriminated between all patients presenting with ADAMTS13 IgG antibodies in the highest quartile. There was a significant difference in the presenting antigen level when comparing those who died and survivors (IgG >77% and died antigen 0.8% vs IgG >77% and survived antigen 2.7%, P = .02).
The highest rate of mortality was seen in patients who presented with both the highest anti-ADAMTS13 antibody levels (IgG >77%) and the lowest ADAMTS13 antigen levels (<1.5%). The mortality for these patients was 27.3% compared with a mortality of 10.2% for those with only 1 of the anti-ADAMTS13 antibody level or the ADAMTS13 antigen level in the most extreme quartile (mortality if anti-ADAMTS13 antibody >77% and ADAMTS13 antigen <1.5% = 27.3%, mortality if either of anti-ADAMTS13 antibody >77% or ADAMTS13 antigen <1.5% but not both, = 10.2%, %; P = .02). The mortality rate for patients with the highest anti-ADAMTS13 antibody levels (IgG >77%) and the lowest ADAMTS13 antigen levels (<1.5%) was also more than 3 times higher for patients with an anti-ADAMTS13 antibody level >77%, but a high ADAMTS13 antigen level (>11%), although this was not found to be statistically significant (mortality if antibody >77% and antigen <1.5% = 27.3% vs mortality if antibody >77% and antigen >11% 7.7%, P = .24) (Table 3). This difference in mortality was not explained by the presence of a strong inhibitory antibody as these were seen in the same frequency in both subgroups (% strong inhibitory antibody if anti-ADAMTS13 antibody >77% and antigen <1.5% = 72.7% vs % strong inhibitory antibody if anti-ADAMTS13 antibody >77% and antigen >11% 76.9%, P > .999).
Mortality when combining extremes of anti-ADAMTS13 IgG antibody and ADAMTS13 antigen results
| Mortality . | Low ADAMTS13 antigen (<1.5%) . | High ADAMTS13 antigen (>11%) . |
|---|---|---|
| High anti-ADAMTS13 antibody (>77%) | 27.3% (n = 33) | 7.7% (n = 13) |
| Inhibitor detected (% strong) | 100% (72.7) | 100% (76.9) |
| Low anti-ADAMTS13 antibody (<20%) | 0% (n = 8) | 5.6% (n = 32) |
| Inhibitor detected (% strong) | 100% (12.5) | 69.4% (5.0) |
| Mortality . | Low ADAMTS13 antigen (<1.5%) . | High ADAMTS13 antigen (>11%) . |
|---|---|---|
| High anti-ADAMTS13 antibody (>77%) | 27.3% (n = 33) | 7.7% (n = 13) |
| Inhibitor detected (% strong) | 100% (72.7) | 100% (76.9) |
| Low anti-ADAMTS13 antibody (<20%) | 0% (n = 8) | 5.6% (n = 32) |
| Inhibitor detected (% strong) | 100% (12.5) | 69.4% (5.0) |
Discussion
Although the understanding of TTP has improved significantly in the past 20 years, acute mortality remains unacceptably high. With novel products such as caplacizumab,26 an anti–VWF humanized nanobody, being trialed and some evidence for intensification of plasma exchange27 in very unwell patients, there is increasing scope for treatment tailored according to individual risk profiles.
This is the largest prospective cohort study of this rare condition reflecting the UK experience in immune-mediated TTP. Many of the demographic findings seen confirm those of previous studies: the condition is seen in disproportionately more women, people of Afro-Caribbean ancestry, and in those who are middle aged. The UK wide mortality rate of 10% is at the lower end of published data,2,9 but there remain discrepancies between centers. There has previously been a lack of consensus on the value of IgG antibody levels in the management of TTP with evidence both for28 and against29 its importance in previous smaller studies. This study confirms their importance in the acute management of TTP with mortality, cardiac involvement and neurological involvement all increased in patients with higher anti-ADAMTS13 IgG antibody levels and the lowest levels of mortality seen in patients with low anti-ADAMTS13 IgG antibody levels. These levels do not, however, fully explain the mortality. For example, we have also found that antibody levels were higher in de novo patients that relapsed but without a statistically significant increase in the death rate. Why some patients with a markedly raised anti-ADAMTS13 IgG antibody level do better than others may be explained by their ADAMTS13 antigen level. Our results show an unequivocal link between antigen levels and a poor outcome with mortality almost 5 times higher for those in the lowest quartile for antigen level compared with the highest quartile. In patients with a high anti-ADAMTS13 IgG antibody level, the median antigen level was 3 times higher in survivors than in those who died; in this same subgroup, mortality was almost 3 times higher in patients with a low antigen compared with those with a high antigen (although we were unable to prove statistical significance). These results, in particular the latter, suggest the presence of inhibitory antibodies is not the primary factor alone affecting mortality; rather, the antigen level is the defining parameter. The reason for this needs further evaluation; it may be that patients with very low antigen levels have increased clearance of circulating ADAMTS13, as previously demonstrated,21 than those with a higher antigen level but this is not picked up because it is below the level detectable with existing ADAMTS13 assays.
This work has confirmed the findings of previous studies10,12-14 suggesting an elevated cardiac troponin and neurological involvement in TTP are poor prognostic indicators. Crucially, more subtle neurological change than previously recognized may be a significant predictor of mortality. In previous studies, cerebral involvement has been variably defined by a broad range of symptoms, including headache, stupor, stroke, seizure, and focal deficiency. Headache is not uncommon in TTP with studies variably estimating that it is seen in 60%30 to 78%2 of patients at presentation, rising to 90% during the course of the TTP episode. In our study, a reduced GCS was associated with an almost 10-fold increase in mortality. Many of the patients included had a GCS of 14/15, with confusion as their only symptom, a sometimes subtle finding that can be overlooked. In our mind, this leaves no doubt on the clinical utility of GCS as a quick bedside test that needs no prior knowledge of TTP to undertake and can be used as a specific method to identify high-risk patients. Similarly, patients with a raised cardiac troponin level had a mortality rate 6 times higher than cardiac troponin values in the normal range and this confirms its importance as a tool in identifying high-risk TTP patients.
There are limitations to this study. It is likely that the overall incidence of TTP is underestimated because the Registry only captures cases in which the diagnosis of TTP was recognized and an appropriate sample taken and sent. This may also mean underrepresentation of more aggressive cases in which the patient has died soon after presentation and before the diagnosis was made. With patients being treated at multiple centers, there is likely to be some variation in treatment between patients that could affect our reported observations. This effect is likely compounded by the broad study period as a greater understanding of the pathophysiology of TTP and the introduction of therapies such as rituximab have meant more aggressive treatment in recent years. In this respect, the overall number of relapsed TTP episodes is small, and although the risk overall risk of organ involvement and mortality appears reduced, statistical differences from the much larger de novo cases have not been reached. This does not suggest, however, that relapsed cases require any less intensive treatment because there still remains a mortality risk as demonstrated.
Regarding study design, it is important to mention that we only undertook 50:50 mixing study testing to confirm the presence of inhibitory antibodies on samples that fell in the most extreme quartiles for antibody or antigen results. This is because mixing studies are a useful screening test for an inhibitor without being specific and undertaken only within those quartiles we were studying to confirm the presence of a strong inhibitory antibody. An alternative approach would have been to use a Bethesda-based assay to measure inhibitors but aside from being cumbersome, the exact meaning of the results has never been formally assessed. Furthermore, the levels of IgG antibodies show a relevant clinical association.
In conclusion, we report that increasing anti-ADAMTS13 IgG antibodies are associated with increased mortality in acute TTP; furthermore, ADAMTS13 antigen levels provide an additional correlate with clinical outcome. Raised cardiac troponin and reduced GCS score are valuable markers of poor prognosis and should be used accordingly in routine clinical practice allowing intensification of therapy in higher risk patients with potential for improved outcomes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Funding was received by unrestricted educational funding from Octapharma and Ablynx (F.A.).
Authorship
Contribution: F.A. wrote the paper and undertook laboratory testing; V.M., S.A., A.C., W.L., R.G., T.B., T.D., N.C., O.C., T.C., K.D., H.G.W., J.J.v.V., K.S., W.T., L.M., Q.A.H., S.B., J.-P.W., D.E., and M.T. collected data and reviewed the manuscript; K.V. and C.V. designed the ADAMTS13 antigen assay and reviewed the manuscript; F.A., C.V., and K.L. undertook laboratory testing; and M.S. was senior author, collected data, and reviewed the manuscript.
Conflict-of-interest disclosure: W.L. received honoraria from Ablynx and Alexion for attendance at advisory boards. M.S. received honoraria from Ablynx, Alexion, Novartis, and Shire for attendance at advisory boards and speaker fees from Octapharma. A.C., J.J.v.V., and R.G. recieved honoraria from Ablynx for advisory board attendance. The remaining authors declare no competing financial interests.
Correspondence: Ferras Alwan, University College London Hospital, Department of Haematology (PA Jessica Blanchard), 307 Euston Rd, London, NW1 2BU, United Kingdom; e-mail: ferras.alwan@nhs.net.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal