Key Points
ACE plays an important physiological role in neutrophil antibacterial activity.
ACE upregulation in mice neutrophils strongly enhances bactericidal activity via increased reduced NAD phosphate oxidase production of ROS.
Abstract
Angiotensin-converting enzyme (ACE) inhibitors are widely used to reduce blood pressure. Here, we examined if an ACE is important for the antibacterial effectiveness of neutrophils. ACE knockout mice or mice treated with an ACE inhibitor were more susceptible to bacterial infection by methicillin-resistant Staphylococcus aureus (MRSA). In contrast, mice overexpressing ACE in neutrophils (NeuACE mice) have increased resistance to MRSA and better in vitro killing of MRSA, Pseudomonas aeruginosa, and Klebsiella pneumoniae. ACE overexpression increased neutrophil production of reactive oxygen species (ROS) following MRSA challenge, an effect independent of the angiotensin II AT1 receptor. Specifically, as compared with wild-type (WT) mice, there was a marked increase of superoxide generation (>twofold, P < .0005) in NeuACE neutrophils following infection, whereas ACE knockout neutrophils decreased superoxide production. Analysis of membrane p47-phox and p67-phox indicates that ACE increases reduced NAD phosphate oxidase activity but does not increase expression of these subunits. Increased ROS generation mediates the enhanced bacterial resistance of NeuACE mice because the enhanced resistance is lost with DPI (an inhibitor of ROS production by flavoenzymes) inhibition. NeuACE granulocytes also have increased neutrophil extracellular trap formation and interleukin-1β release in response to MRSA. In a mouse model of chemotherapy-induced neutrophil depletion, transfusion of ACE-overexpressing neutrophils was superior to WT neutrophils in treating MRSA infection. These data indicate a previously unknown function of ACE in neutrophil antibacterial defenses and suggest caution in the treatment of certain individuals with ACE inhibitors. ACE overexpression in neutrophils may be useful in boosting the immune response to antibiotic-resistant bacterial infection.
Introduction
Neutrophils are an essential component of the innate immune response.1 As first responders, blood neutrophils engulf and kill invading pathogens. Not surprisingly, neutrophil number and functional maturation are tightly regulated by several factors, including cytokines and bioactive peptides.1-3
Activation of neutrophil reduced NAD phosphate (NADPH) oxidase (NOX2) and the subsequent generation of superoxide, hydrogen peroxide, and other reactive oxygen species (ROS) play a central role in the antibacterial activities of neutrophils.2-4 ROS not only kill pathogens in phagosomes, but also activate other important antimicrobial mechanisms, including the release of proteins and fibrils to entrap and kill bacteria, a process called neutrophil extracellular trap (NET) formation.2,3,5,6
Angiotensin-converting enzyme (ACE) is a key component of the renin-angiotensin system, and is responsible for converting angiotensin I to the vasoactive peptide angiotensin II (Ang II).7,8 Millions of patients take ACE inhibitors to treat hypertension and cardiovascular disease. Unlike renin, which is very limited in tissue expression and enzymatic specificity, ACE is expressed in many tissues and is enzymatically promiscuous; in addition to angiotensin I, ACE can cleave many other peptides such as bradykinin, substance P, enkephalins, and several other peptides.9 Because of this, ACE affects diverse biological processes, including renal development, male reproduction, and several aspects of the immune response.8-13 For example, ACE affects the functional maturation of both myeloid and erythroid lineage cells.14
Our laboratory has investigated the role of ACE in monocytic function. We found that overexpression of ACE in monocytes and macrophages upregulates the immune responses of these cells.13-18 Neutrophils and monocytes are derived from a common precursor and share many biological features. This led us to investigate the natural role of ACE in neutrophil function and whether ACE overexpression would enhance neutrophil function. Studying both ACE knockout (KO) mice and a new line of transgenic mice called NeuACE, we found that ACE plays an important physiologic role in the antimicrobial activities of neutrophils. Further, the upregulation of ACE in neutrophils strongly enhances antibacterial immunity in mice. This appears to be the result of a marked increase of NOX2 activity and ROS generation associated with cell activation. Clinically, neutrophil transfusion is used in immunosuppressed patients. The overexpression of ACE in neutrophils endows these cells with a substantially higher capacity to transfer immune resistance to bacterial infection. In contrast, ACE inhibitors appear deleterious to neutrophil function. Given the importance of the innate immune response, these findings raise the possibility of a very novel means of increasing immune resistance.
Methods
Mice
All animal experiment protocols were approved by the Cedars-Sinai Institutional Animal Care and Usage Committee. ACE KO mice were described previously.19 These mice were back-crossed to C57BL/6 mice for 10 or more generations.
To generate NeuACE mice, mouse ACE complementary DNA was modified to contain the mouse c-fms promoter before the transcription start site (see supplemental Figure 3A, available on the Blood Web site).15,20 C57BL/6 mice were then made transgenic for this construct using standard approaches. Screening of several different founder lines identified the NeuACE mouse family, which was unique in overexpressing only ACE in neutrophils. NeuACE mice are distinct from the previously reported ACE 10/10 mice.15 The NeuACE mice were bred to homozygosity for the transgenic construct. All mice were 8 to 12 weeks old. Both male and female mice were used and no phenotypic differences were noted.
Bacterial strains
Methicillin-resistant Staphylococcus aureus (MRSA; strain LAC, US300), green fluorescent protein (GFP)-expressing. S aureus (GFP-Staph., RN4220), Klebsiella pneumoniae (ATCC 10031), and Pseudomonas aeruginosa (ATCC 35032) have been previously described.21,22 They were grown as described in the supplemental Methods.
In vivo MRSA infection
Bacteria were washed twice and resuspended in sterile phosphate buffered saline (PBS). Bacteria were adjusted to the desired concentrations by absorbance assuming an optical density of 600 nm of 0.3 was equivalent to 1 × 108 colony-forming units per milliliter (CFUs/mL). CFU numbers used in each experiment were confirmed by plating. For skin infection, mice were shaved the day before subcutaneous injection of 100 μL PBS per mouse flank at the bacterial doses described in the figures. The lesion size was quantified over the course of 3 days postinfection as described.23 If not indicated otherwise, the mice were euthanized and skin lesions were excised and homogenized in 1 mL of PBS for determination of CFU by serial dilutions. Afterward, the homogenate was centrifuged for 5 minutes and the supernatant was used for cytokine measurement.
For systemic infection, ∼1 × 107 CFU of MRSA was injected into mice through the retro-orbital vein. After 48 hours, blood was drawn via the retro-orbital vein, and, in some experiments, mice were euthanized and splenic single-cell suspensions were prepared for analysis of ACE, myeloperoxidase (MPO), and cell subpopulations.
Other methods
All other methods, such as bone marrow (BM) transplantation, ACE inhibitors, neutrophil isolation and depletion, in vitro blood killing, superoxide, ROS, and MPO measurements, or NET formation are previously published protocols described in detail in supplemental Methods.
Results
ACE is required for normal neutrophil antibacterial activity
We first evaluated ACE expression in neutrophils following MRSA infection because activated neutrophils markedly change the profile of expressed proteins. Wild-type (WT) mice were challenged with MRSA (IV) and, after 2 days, ACE expression was determined using flow cytometry (FCM). In response to acute MRSA stimulation, both blood and splenic neutrophils increased surface ACE expression (Figure 1A).
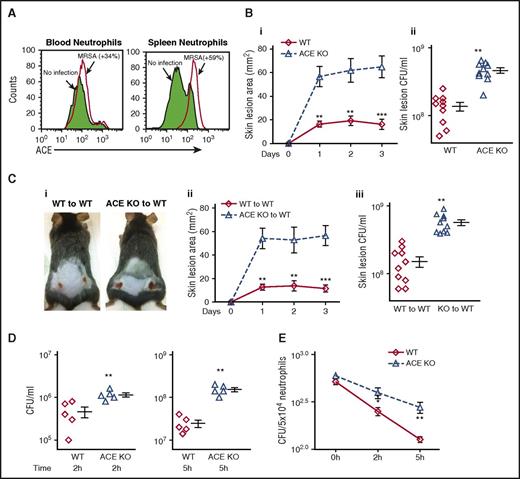
ACE plays a physiological role in antibacterial defense. (A) Neutrophil ACE expression in response to MRSA stimulation. WT mice were injected IV with MRSA. After 48 hours, ACE expression was determined in CD11b+Ly6G+ neutrophils by FCM. The figure is representative of 5 mice. ACE expression was increased in both blood and splenic neutrophils following MRSA infection. (B) Resistance to MRSA infection in WT (◊) and ACE KO (Δ) mice. Groups of mice were infected subcutaneously with MRSA (1 × 107 CFU/mouse flank). (i) Skin lesion area per day postinfection, (ii) Bacterial burden in individual lesions on day 3 postinfection. (C) Bacterial resistance following transplantation of either WT or ACE KO BM. (i) Representative image of skin lesions at day 3 post-infection, (ii) Skin lesion area, (iii) Bacterial burden per lesion. (D) In vitro MRSA clearance by blood from WT mice or ACE KO mice. Following infection with ∼106 CFU/mL MRSA, blood samples from mice were assessed for their ability to eliminate MRSA after 2 or 5 hours of incubation. (E) In vitro intracellular killing of MRSA in neutrophils purified from bone marrow. Intracellular killing was significantly lower in ACE KO compared with WT neutrophils (n = 8/group). *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
ACE plays a physiological role in antibacterial defense. (A) Neutrophil ACE expression in response to MRSA stimulation. WT mice were injected IV with MRSA. After 48 hours, ACE expression was determined in CD11b+Ly6G+ neutrophils by FCM. The figure is representative of 5 mice. ACE expression was increased in both blood and splenic neutrophils following MRSA infection. (B) Resistance to MRSA infection in WT (◊) and ACE KO (Δ) mice. Groups of mice were infected subcutaneously with MRSA (1 × 107 CFU/mouse flank). (i) Skin lesion area per day postinfection, (ii) Bacterial burden in individual lesions on day 3 postinfection. (C) Bacterial resistance following transplantation of either WT or ACE KO BM. (i) Representative image of skin lesions at day 3 post-infection, (ii) Skin lesion area, (iii) Bacterial burden per lesion. (D) In vitro MRSA clearance by blood from WT mice or ACE KO mice. Following infection with ∼106 CFU/mL MRSA, blood samples from mice were assessed for their ability to eliminate MRSA after 2 or 5 hours of incubation. (E) In vitro intracellular killing of MRSA in neutrophils purified from bone marrow. Intracellular killing was significantly lower in ACE KO compared with WT neutrophils (n = 8/group). *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
We next challenged ACE KO and WT mice with a subcutaneous injection of MRSA (107 CFU/mouse flank). Three days postinfection, the mice were euthanized, and the skin lesional area and lesional bacterial burden were measured. ACE KO mice have significantly larger skin lesions (fourfold) and more bacteria per lesion (3.3-fold) compared with WT mice (Figure 1B).
ACE KO mice have systemic differences from WT mice, including low blood pressure. Because of this, we investigated the role of ACE in innate immunity using BM transplantation. WT mice were transplanted with BM from either WT mice or ACE KO mice. Western blot verified successful transplantation (supplemental Figure 1). Eight weeks after transplantation, mice were challenged with subcutaneous MRSA. WT mice transplanted with ACE KO BM exhibited substantially increased skin lesional area (4.9-fold) and lesional bacterial counts (3.5-fold) compared with the transplantation of WT BM (Figure 1C). Thus, these studies indicate an important role of ACE in the innate immune response to MRSA.
To examine the ability of phagocytic cells to kill MRSA, we used an MRSA blood killing assay in which peripheral blood from WT and ACE KO mice was mixed with MRSA ex vivo. Viable bacteria were determined at 2 and 5 hours. Bacterial clearance was significantly lower in the blood of ACE KO mice compared with that of WT mice (Figure 1D). To directly assess the ability of ACE KO neutrophils to kill MRSA, an intracellular killing assay was performed using purified neutrophils from BM. MRSA was added at a multiplicity of infection (MOI) of 10, and phagocytosis continued for 20 minutes. Extracellular bacteria were then killed and the number of viable bacteria as CFU was determined after cell lysis. Intracellular bacterial killing by neutrophils from WT mice was substantially better than neutrophils from ACE KO mice (Figure 1E). The peripheral blood of ACE KO animals has equivalent numbers of peripheral neutrophils and other immune cells as compared with WT mice (supplemental Figure 2).
ACE overexpression in neutrophils enhanced antibacterial activity
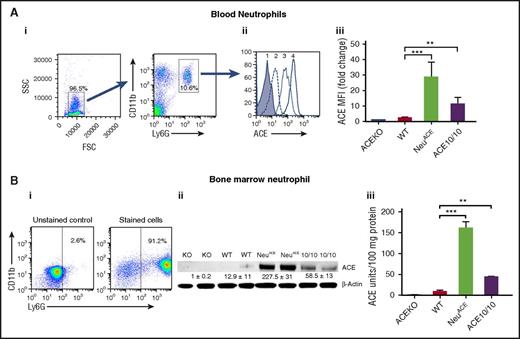
Because the lack of ACE expression in neutrophils and other hematopoietic cells was associated with a diminished innate immune response, we also investigated the phenotype of animals overexpressing ACE in neutrophils. C57Bl/6 mice were made transgenic for a construct in which ACE complementary DNA is under the control of the c-fms promoter (supplemental Figure 3). This approach does not alter the natural ACE gene in the transgenic mice. Founder mice were screened for high neutrophil ACE expression. Eventually 1 family, termed NeuACE, was bred to homozygosity for the transgenic construct. By western blot, FCM, and ACE activity measurement, NeuACE neutrophils express approximately 12- to 18-fold higher levels of ACE compared with equivalent WT cells (Figure 2). As an example, NeuACE neutrophils make 17.6-fold more ACE than equivalent WT cells by ACE activity assay. Other leukocytes (monocytes, macrophages, dendritic cells, B cells, and T cells) and organs (lung, kidney, liver, and spleen) of NeuACE mice were not significantly different in ACE expression from WT (supplemental Figures 4-8). The percentages of blood and BM neutrophils were not statistically different between NeuACE mice and WT (supplemental Figure 9).
Characterization of NeuACEneutrophils. (A) FCM analysis of ACE expression by blood neutrophils. (i) Dot plots show the approach for the profiling of neutrophils. Neutrophils were defined as CD11b+Ly6G+. FSC, forward scatter; SSC, side scatter. (ii) Histogram of flow cytometry results using anti-ACE antibody and neutrophils from ACE KO (peak 1), WT (2), ACE 10/10 (3), and NeuACE mice (4). (iii) Analysis of ACE expression by ACE KO, WT, ACE 10/10, and NeuACE neutrophils. Data are presented as mean fluorescence intensity (MFI) (n = 4/group). (B) ACE protein expression in neutrophils. Neutrophils were purified from bone marrow using Percoll gradient centrifugation. Flow cytometric analysis after staining cells with CD11b and Ly6G showed that approximately 90% of the cells were neutrophils. Purified neutrophils from ACE KO, WT, NeuACE, and ACE 10/10 mice were lysed in ACE assay buffer. ACE expression was determined by western blot analysis (ii) and by ACE catalytic activity (iii). ACE 10/10 mice were previously created by gene targeting of the ACE gene.15 These mice overexpress ACE in myelomonocytic cells. By enzyme assay, NeuACE neutrophils make 3.7-fold more ACE than ACE 10/10 neutrophils. **P ≤ .005, ***P ≤ .0005, KO-ACE KO, 10/10-ACE10/10.
Characterization of NeuACEneutrophils. (A) FCM analysis of ACE expression by blood neutrophils. (i) Dot plots show the approach for the profiling of neutrophils. Neutrophils were defined as CD11b+Ly6G+. FSC, forward scatter; SSC, side scatter. (ii) Histogram of flow cytometry results using anti-ACE antibody and neutrophils from ACE KO (peak 1), WT (2), ACE 10/10 (3), and NeuACE mice (4). (iii) Analysis of ACE expression by ACE KO, WT, ACE 10/10, and NeuACE neutrophils. Data are presented as mean fluorescence intensity (MFI) (n = 4/group). (B) ACE protein expression in neutrophils. Neutrophils were purified from bone marrow using Percoll gradient centrifugation. Flow cytometric analysis after staining cells with CD11b and Ly6G showed that approximately 90% of the cells were neutrophils. Purified neutrophils from ACE KO, WT, NeuACE, and ACE 10/10 mice were lysed in ACE assay buffer. ACE expression was determined by western blot analysis (ii) and by ACE catalytic activity (iii). ACE 10/10 mice were previously created by gene targeting of the ACE gene.15 These mice overexpress ACE in myelomonocytic cells. By enzyme assay, NeuACE neutrophils make 3.7-fold more ACE than ACE 10/10 neutrophils. **P ≤ .005, ***P ≤ .0005, KO-ACE KO, 10/10-ACE10/10.
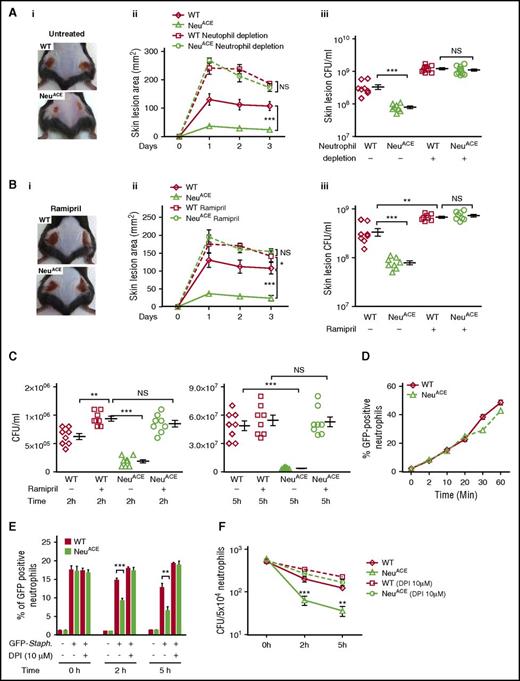
Experiments tested the response of NeuACE mice to subcutaneous MRSA injection (2 × 108 CFU/mouse flank). Three days after infection, NeuACE mice had significantly smaller skin lesions (24 ± 1 mm2) and fewer bacteria (7.8 × 107 ± 7E6 CFU/mL) compared with WT mice (lesion area, 107.6 ± 16 mm2; bacterial count, 3.4 × 108 ± 6E7 CFU/mL) (Figure 3A, P < .0005). To confirm that this difference was due to neutrophils, both NeuACE and WT mice were infected with MRSA following anti-polymorphonuclear neutrophil antibody-induced neutropenia. In the absence of neutrophils, the response to MRSA was not different between NeuACE and WT mice. We also studied MRSA infection in NeuACE and WT mice pretreated for 7 days with the ACE inhibitor ramipril. The inhibitor was continued during the 3-day experiment. ACE blockade significantly increased the lesional area and the bacterial burden in both WT and NeuACE mice. It also eliminated any differences between the 2 strains (Figure 3B). These data suggest that ACE enzymatic activity is important for neutrophil antibacterial activity in both NeuACE and WT mice.
ACE overexpression in neutrophils enhances antibacterial resistance in mice. (A) Resistance to MRSA infection in WT and NeuACE mice. Mice were infected subcutaneously with MRSA (2 × 108 CFU/mouse flank) under basal and neutrophil-depleted conditions. For neutrophil depletion, mice were administered anti-mouse polymorphonuclear neutrophil antibody (250 µg/day/mouse) beginning 1 day before MRSA infection and continuing until the end of the experiment. (i) Representative image of skin lesions at day 3 postinfection, (ii) skin lesion area, and (iii) bacterial burden (CFU) per lesion. (B) Similar experiments were conducted with mice treated with ramipril (36.3 mg/L in drinking water) for 1 week before MRSA infection. (C) Blood from NeuACE mice cleared MRSA more effectively than blood from WT mice at both 2 and 5 hours. Blood samples were infected with MRSA at ∼106 CFU/mL. However, when this assay was performed with blood from mice pretreated with ramipril for 7 days, no difference in bacterial clearance was observed between the 2 groups. (D) Bacterial phagocytosis (internalization) was determined using GFP-Staph. Bone marrow neutrophils were purified and infected with GFP-Staph. (MOI ∼20). The time-dependent percentage of GFP+ neutrophils was determined by FCM (n = 4/group). (E) Measurement of neutrophil phagocytic killing. Following 20 minutes of phagocytosis (MOI ∼10, considered 0 time) and killing extracellular bacteria with gentamycin (400 µg/mL), the loss of GFP from neutrophils was determined by FCM. This parallels the killing of ingested bacteria (n = 4/group). (F) Intracellular killing of MRSA in bone marrow neutrophils. After 20 minutes of phagocytosis (MOI ∼10, considered 0 time), extracellular bacteria were killed with gentamycin and then bacterial survival was determined by CFU counting at 0, 2, and 5 hours (n = 4/group). *P ≤ .05, **P ≤ .005, ***P ≤ .0005. NS, nonsignificant.
ACE overexpression in neutrophils enhances antibacterial resistance in mice. (A) Resistance to MRSA infection in WT and NeuACE mice. Mice were infected subcutaneously with MRSA (2 × 108 CFU/mouse flank) under basal and neutrophil-depleted conditions. For neutrophil depletion, mice were administered anti-mouse polymorphonuclear neutrophil antibody (250 µg/day/mouse) beginning 1 day before MRSA infection and continuing until the end of the experiment. (i) Representative image of skin lesions at day 3 postinfection, (ii) skin lesion area, and (iii) bacterial burden (CFU) per lesion. (B) Similar experiments were conducted with mice treated with ramipril (36.3 mg/L in drinking water) for 1 week before MRSA infection. (C) Blood from NeuACE mice cleared MRSA more effectively than blood from WT mice at both 2 and 5 hours. Blood samples were infected with MRSA at ∼106 CFU/mL. However, when this assay was performed with blood from mice pretreated with ramipril for 7 days, no difference in bacterial clearance was observed between the 2 groups. (D) Bacterial phagocytosis (internalization) was determined using GFP-Staph. Bone marrow neutrophils were purified and infected with GFP-Staph. (MOI ∼20). The time-dependent percentage of GFP+ neutrophils was determined by FCM (n = 4/group). (E) Measurement of neutrophil phagocytic killing. Following 20 minutes of phagocytosis (MOI ∼10, considered 0 time) and killing extracellular bacteria with gentamycin (400 µg/mL), the loss of GFP from neutrophils was determined by FCM. This parallels the killing of ingested bacteria (n = 4/group). (F) Intracellular killing of MRSA in bone marrow neutrophils. After 20 minutes of phagocytosis (MOI ∼10, considered 0 time), extracellular bacteria were killed with gentamycin and then bacterial survival was determined by CFU counting at 0, 2, and 5 hours (n = 4/group). *P ≤ .05, **P ≤ .005, ***P ≤ .0005. NS, nonsignificant.
Next, we examined the ability of peripheral blood from NeuACE mice to kill MRSA in vitro (Figure 3C). At both 2 and 5 hours after addition of MRSA, bacterial titers were significantly lower in blood from NeuACE mice compared with blood from WT mice. However, when this experiment was performed using blood from mice pretreated for 7 days with ramipril, blood from NeuACE mice was less effective in clearing bacteria and there was now no difference between blood from NeuACE and WT mice.
To investigate whether ACE itself directly participates in bacterial killing, in vitro blood killing was assessed using blood pretreated for only 30 minutes with ramipril. Here, there was no pretreatment of the mice. When blood from WT or NeuACE was then challenged with MRSA, there was no difference in killing between the samples with or without the acute addition of ramipril (supplemental Figure 10). Additionally, we found no direct effects of pure mouse ACE (affinity purified from mice kidney and lung) on the growth of MRSA (supplemental Figure 11). Thus, ACE activity itself does not participate in the killing of bacteria.
Finally, we investigated the effectiveness of NeuACE and WT blood when challenged with either P aeruginosa or K pneumoniae. Similar to the results with MRSA, substantially better in vitro killing of these pathogens was observed by NeuACE blood compared with WT (supplemental Figure 12A-B). Together, data in Figures 1-3 indicate that ACE catalytic activity affects the physiologic function of neutrophils. Further, ACE overexpression enhances neutrophil antibacterial response against several bacterial species.
ACE affects neutrophil oxidative response
To further compare bacterial killing in NeuACE and WT neutrophils, we measured phagocytosis. BM neutrophils were mixed with S aureus producing GFP (MOI ∼20). Bacterial internalization over time was determined by FCM. We found no difference in bacterial internalization between NeuACE and WT neutrophils at 0 to 20 minutes (Figure 3D). At 30 to 60 minutes, a slightly lower number of bacteria was measured in NeuACE neutrophils compared with WT neutrophils, but these differences were not significant.
To investigate phagocytic killing, neutrophils were allowed to phagocytize bacteria (MOI ∼10) for 30 minutes. Extracellular bacteria were then killed with gentamicin and the loss of GFP signal was followed by FCM. The loss of GFP, which parallels the killing of ingested S aureus,24 was significantly more rapid in NeuACE than in WT neutrophils at both 2 and 5 hours (Figure 3E). To more precisely quantitate intracellular killing of MRSA, we counted intracellular CFU at 2 and 5 hours. This also showed a significantly higher rate of bacterial killing in NeuACE neutrophils compared with WT neutrophils (Figure 3F). When these experiments were repeated in the presence of DPI (an inhibitor of ROS production by flavoenzymes), we now saw no difference in bacterial killing efficiency between NeuACE and WT neutrophils.
The production of ROS is very important for neutrophil antimicrobial activity.2,25 To directly examine whether the ROS response in neutrophils changes with ACE status, we used an assay in which neutrophil oxidation of the intracellular indicator DHR 123 was determined by FCM under normal and activated conditions. To activate cells, we challenged mice IV with MRSA for 2 days. There was significantly increased oxidation of DHR 123 in NeuACE neutrophils compared with WT, indicating higher production of ROS in the NeuACE cells (Figure 4A).
ACE increases neutrophil oxidative response. (A) ROS production in WT and NeuACE neutrophils under basal conditions and 48 hours after MRSA infection. ROS production by neutrophils was assessed by FCM analysis of DHR 123 oxidation. ROS production was significantly higher in NeuACE vs WT neutrophils. (B) ROS inhibition by DPI (3 mg/kg per day, intraperitoneal [IP]) eliminated the increased bacterial resistance of NeuACE mice. DPI injection was started 1 day before subcutaneous MRSA infection (1 × 108 CFU/mouse flank) and continued until the end of the experiment. (i) Skin lesion area, (ii) Bacterial counts (CFU) in lesions after 3 days. (C) Superoxide production was measured by SOD-inhibitable cytochrome c reduction. Neutrophils were purified from bone marrow and challenged with MRSA in vitro (n = 5/group). (D) Upon activation, the level of NOX2 subunits was measured by western blotting of neutrophil membranes. Neutrophils were purified from bone marrow and activated with MRSA for 15 minutes, and then membranes were isolated using a kit from ThermoFisher. (E) Expression of NOX2 subunits was determined by western blotting of total neutrophil protein lysate. (F) p47-phox phosphorylation (p-Ser345) was determined in neutrophil membranes by western blot analysis. Neutrophils were purified from bone marrow and activated with MRSA (MOI ∼10): (i) groups without MRSA infection (0 minutes) were used as a control, (ii) phospho-p47-phox at 5 minutes after MRSA infection, (iii) phospho-p47-phox at 15 minutes after MRSA infection, (iv) groups with ACE inhibition by ramipril. (G) Effect of gp91 ds-tat, a specific NOX2 inhibitor, on blood killing of MRSA. Blood samples were treated with gp91 ds-tat peptide for 90 minutes (5 μM), and then infected with MRSA at ∼106 CFU/mL. NOX2 inhibition eliminated the difference between WT and NeuACE bacterial killing. (D-G) Data are representative of at least 4 mice in each group. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
ACE increases neutrophil oxidative response. (A) ROS production in WT and NeuACE neutrophils under basal conditions and 48 hours after MRSA infection. ROS production by neutrophils was assessed by FCM analysis of DHR 123 oxidation. ROS production was significantly higher in NeuACE vs WT neutrophils. (B) ROS inhibition by DPI (3 mg/kg per day, intraperitoneal [IP]) eliminated the increased bacterial resistance of NeuACE mice. DPI injection was started 1 day before subcutaneous MRSA infection (1 × 108 CFU/mouse flank) and continued until the end of the experiment. (i) Skin lesion area, (ii) Bacterial counts (CFU) in lesions after 3 days. (C) Superoxide production was measured by SOD-inhibitable cytochrome c reduction. Neutrophils were purified from bone marrow and challenged with MRSA in vitro (n = 5/group). (D) Upon activation, the level of NOX2 subunits was measured by western blotting of neutrophil membranes. Neutrophils were purified from bone marrow and activated with MRSA for 15 minutes, and then membranes were isolated using a kit from ThermoFisher. (E) Expression of NOX2 subunits was determined by western blotting of total neutrophil protein lysate. (F) p47-phox phosphorylation (p-Ser345) was determined in neutrophil membranes by western blot analysis. Neutrophils were purified from bone marrow and activated with MRSA (MOI ∼10): (i) groups without MRSA infection (0 minutes) were used as a control, (ii) phospho-p47-phox at 5 minutes after MRSA infection, (iii) phospho-p47-phox at 15 minutes after MRSA infection, (iv) groups with ACE inhibition by ramipril. (G) Effect of gp91 ds-tat, a specific NOX2 inhibitor, on blood killing of MRSA. Blood samples were treated with gp91 ds-tat peptide for 90 minutes (5 μM), and then infected with MRSA at ∼106 CFU/mL. NOX2 inhibition eliminated the difference between WT and NeuACE bacterial killing. (D-G) Data are representative of at least 4 mice in each group. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
To directly compare the in vivo importance of ROS production to phagocytic killing, NeuACE and WT mice were challenged with subcutaneous MRSA, but animals were now treated with DPI. Under these conditions, both strains showed equivalent skin lesion area and bacterial burden (Figure 4B). Thus, both in vitro and in vivo results show that increased ROS production by NeuACE neutrophils contributes to their better killing of bacteria.
NOX2 is the major source of oxidants in neutrophils upon activation
In neutrophils, NOX2 oxidase and MPO plays an important role in ROS-mediated bacterial killing.26 We examined NOX2 activity by measuring superoxide production using a cytochrome c reduction assay. Compared with WT, there was a highly significantly increase of superoxide generation (> twofold, P < .0005) in NeuACE neutrophils following MRSA infection (Figure 4C), suggesting ACE expression directly affects NOX2 activity. We next examined the level of NOX2 subunits in the neutrophil membrane fraction since upon activation, cytosolic subunits of NOX2 assemble with membrane gp91-phox.4 When neutrophils were exposed to MRSA for 15 minutes, we found a significantly higher level of NADPH oxidase subunits p47-phox and p67-phox (two- to threefold, P < .05) in NeuACE neutrophil membranes compared with WT, whereas no significant difference was observed in abundance of gp91-phox (Figure 4D). When we compared total cellular expression of NADPH oxidase subunits, we found no significant difference between NeuACE and WT neutrophils (Figure 4E), which indicates that ACE most probably affects NOX2 activation rather than protein expression. Analysis of superoxide production by neutrophils from ACE KO mice showed the opposite effect; these cells made significantly less superoxide than WT cells in response to phorbol 12-myristate 13-acetate (PMA) stimulation (supplemental Figure 13).
Because phosphorylation of p47-phox is critical for activation of NOX2, we measured membrane p47-phox phosphorylation (pSer345) upon activation with MRSA. Compared with WT neutrophils, p47-phox phosphorylation was significantly higher in NeuACE neutrophils at 15 minutes after MRSA-mediated activation (Figure 4Fiii, P < 002). In contrast, at an earlier time point (5 minutes) and in control groups (0 minutes) with no MRSA infection, we found no significant difference in p47-phox phosphorylation. ACE inhibition with ramipril eliminated the difference in membrane phospho-p47-phox levels between NeuACE and WT cells (Figure 4Fiv).
We assessed specific MPO activity and total peroxidase activity in MRSA-activated neutrophils. No difference was observed between WT and NeuACE neutrophils (supplemental Figure 14). To directly study the role of NOX2 in enhanced antibacterial activity of NeuACE neutrophils, we measured MRSA killing efficiency of blood killing in the presence of the highly specific NOX2 blocker, gp91 ds-tat.27 NOX2 inhibition with this peptide totally reverted NeuACE neutrophils to a WT phenotype as measured by blood killing of MRSA (Figure 4G).
We previously published a mouse model called ACE 10/10 in which ACE is overexpressed in monocytes, macrophages, and other myelomonocytic cells.15 Evaluation of ACE 10/10 macrophages showed that these cells also overexpress superoxide in response to PMA (supplemental Figure 5viii). Thus, increased ACE activity leads to enhanced superoxide production by neutrophils and macrophages.
ACE influences NET formation and cytokine production
Other than phagocytic killing, neutrophils kill pathogens by releasing NET.28,29 An important stimulus for NET formation is granulocytic ROS production.5,6,29 To study NET formation in NeuACE mice, BM neutrophils were purified and then treated with PMA. NET formation was analyzed by fluorescent microscopy following DNA staining. NeuACE neutrophils showed a higher rate of NET formation compared with WT (Figure 5A). Further, MRSA-induced NET formation was quantitated using an assay that specifically measured NET-associated neutrophil elastase. The amount of NET-associated elastase paralleled the release of double-stranded DNA, a major component of NETs.30,31 NeuACE neutrophils release significantly more NET-associated elastase than equivalent WT cells (Figure 5B). The inhibition of ROS by DPI reduced NET-associated elastase release by NeuACE neutrophils, eliminating the difference between these cells and WT neutrophils (Figure 5B). ACE inhibition by ramipril also abrogated the higher rate of NET formation in NeuACE mice (Figure 5C).
ACE increases neutrophil NET formation and IL-1β production. (A) NET formation was measured by fluorescent microscopy. Neutrophils were purified to 98% using magnetic beads and a kit from Stem Cell. They were then cultured and stimulated with PMA (50 nM) for 3 hours. After staining with Cytox green, pictures were taken at ×20 using fluorescent microscope. (B) Elastase release by WT and NeuACE neutrophils. WT and NeuACE neutrophils were purified from blood by gradient centrifugation. Some mice were treated with DPI (3 mg/kg/single dose) for 1 day before neutrophil collection and the purified cells were treated again for 1 hour (10 µM) before MRSA challenge. In all groups, cells were treated with MRSA for 4 hours before NET-associated neutrophil elastase was measured by ELISA. n = 5/group. (C) Following ACE inhibition by ramipril, NET formation was determined by elastase-based ELISA, as described previously. (D) Following 3 days of subcutaneous skin infection with MRSA, NeuACE mice had increased levels of IL-1β in skin lesions compared with WT; n = 8/group. The difference in IL-1β production was abolished by treatment with DPI (3 mg/kg per day, i.p.) beginning 1 day before infection and continuing throughout the experiment; n = 8/group. (E) To assess IL-1β production in vitro, bone marrow neutrophils from WT and NeuACE mice were cultured with MRSA (5 × 106 neutrophils/well, 10 CFU MRSA/neutrophil) for 12 hours. Total IL-1β (cells plus supernatant) was determined by ELISA; n = 5/group. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
ACE increases neutrophil NET formation and IL-1β production. (A) NET formation was measured by fluorescent microscopy. Neutrophils were purified to 98% using magnetic beads and a kit from Stem Cell. They were then cultured and stimulated with PMA (50 nM) for 3 hours. After staining with Cytox green, pictures were taken at ×20 using fluorescent microscope. (B) Elastase release by WT and NeuACE neutrophils. WT and NeuACE neutrophils were purified from blood by gradient centrifugation. Some mice were treated with DPI (3 mg/kg/single dose) for 1 day before neutrophil collection and the purified cells were treated again for 1 hour (10 µM) before MRSA challenge. In all groups, cells were treated with MRSA for 4 hours before NET-associated neutrophil elastase was measured by ELISA. n = 5/group. (C) Following ACE inhibition by ramipril, NET formation was determined by elastase-based ELISA, as described previously. (D) Following 3 days of subcutaneous skin infection with MRSA, NeuACE mice had increased levels of IL-1β in skin lesions compared with WT; n = 8/group. The difference in IL-1β production was abolished by treatment with DPI (3 mg/kg per day, i.p.) beginning 1 day before infection and continuing throughout the experiment; n = 8/group. (E) To assess IL-1β production in vitro, bone marrow neutrophils from WT and NeuACE mice were cultured with MRSA (5 × 106 neutrophils/well, 10 CFU MRSA/neutrophil) for 12 hours. Total IL-1β (cells plus supernatant) was determined by ELISA; n = 5/group. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
Neutrophils also attack pathogens using cytokines that not only enhance neutrophil recruitment but also act to recruit and activate other immune cells.21,32,33 We examined production of interleukin 1β (IL-1β) as it is a critical cytokine for antibacterial activity.21,33 Three days after subcutaneous MRSA inoculation, IL-1β levels in skin lesions were measured by enzyme-linked immunosorbent assay (ELISA). Lesions from NeuACE mice contained significantly more IL-1β than equivalent lesions from WT mice (Figure 5D). When the same experiment was performed in mice treated with DPI, the difference between NeuACE and WT mice disappeared. This same experiment was also performed with mice depleted of neutrophils, or with mice treated for 1 week with ramipril. Now, there was no difference in IL-1β production between NeuACE and WT mice (supplemental Figure 15).
To directly investigate granulocyte production of IL-1β, neutrophils were highly purified from the BM of NeuACE and WT mice (>98% by FCM). These cells were exposed to MRSA in vitro. After 8 hours, cells were lysed and IL-1β within the lysate (cells plus supernatant) was assessed by ELISA. Again, this experiment showed that neutrophils from NeuACE mice expressed significantly more IL-1β than WT when exposed to MRSA (Figure 5E).
Does the Ang II-AT1 receptor pathway regulate ACE-induced ROS production in neutrophils?
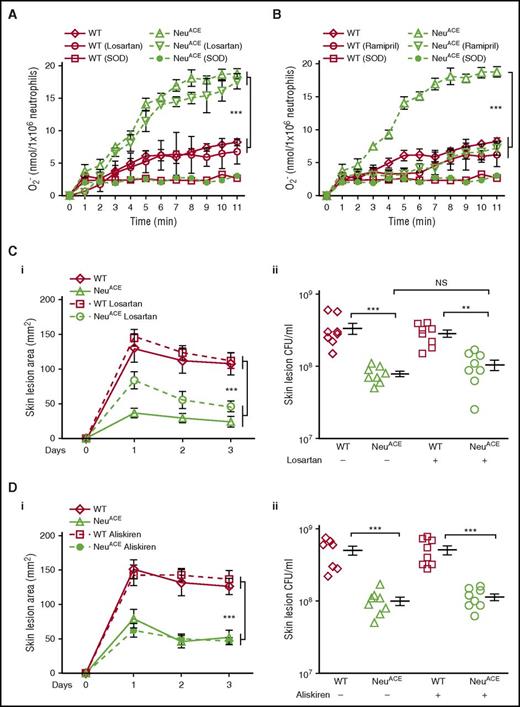
Ang II is an important product of ACE that exerts most effects following binding to cell surface AT1 receptors.34,35 The Ang II/AT1 receptor pathway is known to induce ROS in the cardiovascular system and in circulating leukocytes.36,37 To investigate whether this pathway influences ROS production in NeuACE neutrophils, we analyzed superoxide production in these and WT neutrophils in the presence of the AT1 receptor antagonist losartan. Experiments were also performed in the presence or absence of the ACE inhibitor ramipril. Losartan did not block superoxide generation in NeuACE cells (Figure 6A). This was in contrast to ACE inhibition by ramipril, which reduced superoxide production by NeuACE cells and abolished the difference between NeuACE and WT cells (Figure 6B). Similarly, increased peroxide production in response to MRSA was completely eliminated by ramipril, but was not affected by losartan (supplemental Figure 16). In addition, we did not find any difference in the blood levels of Ang II between WT and NeuACE mice (supplemental Figure 17). These data indicate that increased ROS production in NeuACE neutrophils exposed to MRSA is not due to Ang II–mediated binding to the AT1 receptor, but is due to ACE activity.
The angiotensin II AT1 receptor is not the major pathway for increased bacterial resistance in NeuACEmice. (A-B) Superoxide production was measured by cytochrome c reduction in neutrophils isolated from bone marrow following a 5-hour in vitro challenge with MRSA (1 × 106 CFU/mL). As a control, samples were treated with 15 µg/mL SOD to eliminate superoxide. (A) Some mice were pretreated with the AT1 receptor antagonist losartan for 1 week (600 mg/L in drinking water) and during superoxide measurements (100 µM). (B) Other mice were pretreated with the ACE inhibitor ramipril for 1 week (36.3 mg/L in drinking water) and during superoxide measurement (1 µM). In both panels A and B, n = 5/group. (C) MRSA skin infection with losartan treatment. Mice were infected subcutaneously with MRSA (2 × 108 CFU/mouse flank). Some mice were treated with losartan as described in panel A. The drug was continued during the experiment. (i) Skin lesion area, (ii) bacterial burden at day 3. (D) MRSA skin infection with aliskiren treatment. Mice were treated IP with the renin inhibitor aliskiren for 5 days (25 mg/kg per day, 1 dose/day) and then infected with MRSA as in panel C. Aliskiren was continued during the experiment. (i) Skin lesion area, (ii) bacterial burden at day 3. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
The angiotensin II AT1 receptor is not the major pathway for increased bacterial resistance in NeuACEmice. (A-B) Superoxide production was measured by cytochrome c reduction in neutrophils isolated from bone marrow following a 5-hour in vitro challenge with MRSA (1 × 106 CFU/mL). As a control, samples were treated with 15 µg/mL SOD to eliminate superoxide. (A) Some mice were pretreated with the AT1 receptor antagonist losartan for 1 week (600 mg/L in drinking water) and during superoxide measurements (100 µM). (B) Other mice were pretreated with the ACE inhibitor ramipril for 1 week (36.3 mg/L in drinking water) and during superoxide measurement (1 µM). In both panels A and B, n = 5/group. (C) MRSA skin infection with losartan treatment. Mice were infected subcutaneously with MRSA (2 × 108 CFU/mouse flank). Some mice were treated with losartan as described in panel A. The drug was continued during the experiment. (i) Skin lesion area, (ii) bacterial burden at day 3. (D) MRSA skin infection with aliskiren treatment. Mice were treated IP with the renin inhibitor aliskiren for 5 days (25 mg/kg per day, 1 dose/day) and then infected with MRSA as in panel C. Aliskiren was continued during the experiment. (i) Skin lesion area, (ii) bacterial burden at day 3. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
To study the role of the Ang II/AT1 axis in vivo, groups of NeuACE and WT mice were treated for 1 week with losartan or with the renin inhibitor aliskiren. Both drugs were effective as measured by a reduction of blood pressure (supplemental Figure 18). The mice were then challenged with subcutaneous MRSA. Neither losartan nor aliskiren affected the lesion size or bacterial counts of NeuACE or WT mice compared with equivalent mice not treated with the drugs (Figure 6C-D). Similarly, the treatment of WT mice with Ang II for several days also showed no effects on MRSA skin lesion size, lesional bacteria counts, or blood killing of MRSA (supplemental Figure 19). Thus, these data indicate that Ang II is not responsible for the neutrophil antibacterial activity in NeuACE mice. Further, blocking other known ACE-mediated peptide pathways, such as bradykinin/B2R, substance p/NK1R, and Ac-SDKP, had no effects on bacterial killing in mice (supplemental Figure 20).
NeuACE neutrophil therapy is efficacious in controlling bacterial infection
We explored using NeuACE neutrophil therapy for controlling MRSA infection in neutropenic mice. These studies were designed to mimic patients who were neutropenic from chemotherapy and receive neutrophil transfusions to combat infection. Mice were administered a single intraperitoneal dose of cyclophosphamide (230 mg/kg) that was sufficient to render them neutropenic for 4 days (supplemental Figure 21). One day after cyclophosphamide, the mice were infected subcutaneously with MRSA, and 1 day after this, the mice received an IV transfusion of neutrophils purified from the BM of either NeuACE or WT mice. Mice without neutrophil transfer served as controls. At 3 days postinfection, there was no substantial difference in lesion size between mice treated with WT neutrophils or NeuACE neutrophils (Figure 7A). In contrast, the bacterial burden was affected (Figure 7B). Specifically, although the transfer of WT neutrophils into neutropenic mice reduced the bacterial burden, the transfer of NeuACE neutrophils was far more effective and reduced the average lesional bacterial counts to only 1/50th that observed in the absence of neutrophil transfer (Figure 7B).
Transfer of NeuACEneutrophils into immunosuppressed mice increased resistance to MRSA infection. WT mice were made neutropenic with cyclophosphamide (230 mg per mouse, single IP dose). Mice were divided into 3 groups, and 1 day after drug injection, the mice were challenged subcutaneously with MRSA (3 × 106 CFU). Two days after drug injection, mice received 107 neutrophils of IV purified from bone marrow of either WT or NeuACE mice. One group of mice received PBS only. (A) Skin lesion area and (B) bacterial counts in skin lesions (B) were measured 3 days after MRSA infection. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
Transfer of NeuACEneutrophils into immunosuppressed mice increased resistance to MRSA infection. WT mice were made neutropenic with cyclophosphamide (230 mg per mouse, single IP dose). Mice were divided into 3 groups, and 1 day after drug injection, the mice were challenged subcutaneously with MRSA (3 × 106 CFU). Two days after drug injection, mice received 107 neutrophils of IV purified from bone marrow of either WT or NeuACE mice. One group of mice received PBS only. (A) Skin lesion area and (B) bacterial counts in skin lesions (B) were measured 3 days after MRSA infection. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.
Discussion
We describe a new line of mice in which ACE is selectively overexpressed in granulocytes. Granulocytes are the first line of defense against acute bacterial infection.1,2 We now present both in vitro and in vivo evidence that the innate response to acute MRSA infection is increased in mice overexpressing ACE in neutrophils. In contrast, mice lacking ACE activity due to genetic means or because of ACE inhibitors showed the oppose effect: reduced superoxide production and increased susceptibility to infection. Indeed, the comparison of 3 groups of neutrophils (cells lacking ACE expression, cells having WT levels of ACE, and cells overexpressing ACE) showed a direct relationship in which increasing ACE expression is increasingly advantageous in vivo and in in vitro killing by blood, a common assay used to assess neutrophil function. Not only is blood killing of MRSA augmented by ACE overexpression, but a similar analysis of P aeruginosa and K pneumoniae also showed increased killing.
A major question is how does ACE enhance the immune response? Experiments in which an ACE inhibitor was acutely added directly to the blood killing assays showed no effects. In contrast, 7 days’ exposure of mice to an ACE inhibitor, a time sufficient to affect newly formed neutrophils, was associated with significantly diminished bacterial killing. These experiments suggest that it is ACE catalytic activity that is important for the effect of this protein. However, ACE does not directly participate in bacterial killing but probably modifies the phenotype of neutrophils to enhance their immune effectiveness. Granulocytes overexpressing ACE produce more ROS upon activation. This plays a very important role in the increased effectiveness of these neutrophils, as indicated by the effects of the ROS inhibitor DPI. The ACE-overexpressing neutrophils also produce a better NET response and produce increased levels of the pro-inflammatory cytokine IL-1β. These may be downstream effects of increased ROS expression, as indicated by the elimination of the NeuACE advantage following administration of DPI.
In neutrophils, NOX2 is the major source of oxidation. Activation and consequent superoxide production by NOX2 requires assembly of cytosolic subunits with NOX2 in the cell membrane.38,39 Our results showed increased accumulation of cytosolic subunits p47-phox and p67-phox in the membrane of NeuACE neutrophils compared with WT following activation with MRSA. Importantly, we found no difference in the total expression of these subunits in NeuACE and WT neutrophils, indicating ACE enhances activation rather than increases expression of these subunits. It is known that p47-phox activates through phosphorylation and that it not only self-recruits, but also recruits other cytosolic subunits to the membrane, including p67-phox and p40-phox.38 As expected, a higher level of membrane p47-phox in NeuACE neutrophils was also associated with higher levels of phosphorylation. ACE inhibition by ramipril eliminated the difference between NeuACE and WT neutrophils in membrane-associated phospho-p47-phox (pSer345). How ACE influences p47-phox phosphorylation needs to be investigated further. For example, we have not yet determined that status of other p47-phox phosphorylation sites or the kinases responsible for increased phosphorylation. Further, we have not characterized natural scavengers of superoxide, such as superoxide dismutase (SOD) activity, in NeuACE mice.
In the cardiovascular system, Ang II is intimately associated with blood pressure regulation.9,34,35 In mice, ROS plays an important role in Ang II–mediated hypertension. Specifically, Ang II is no longer able to induce hypertension if ROS production is pharmacologically or genetically blocked.36,40,41 What is particularly interesting in our studies is that analysis of the role of ACE vs the AT1 receptor (Ang II) indicates that the enhanced response to infection is independent of AT1 effects but fully dependent on ACE catalytic activity. Indeed, data using the renin inhibitor aliskiren argue that no angiotensin peptide is mediating the NeuACE phenotype. We also found that blocking 3 other well-studied ACE-mediated peptide pathways (bradykinin/B2R, substance p/NK1R, and Ac-SDKP) showed no effects on the control of MRSA infection in mice. At present, we do not know the peptides that stimulate increased ROS production following immune activation.
Enhanced susceptibility to infection is observed in patients undergoing chemotherapy.42,43 Such patients become neutropenic with reduced resistance to infection. This can be treated by the transfusion of exogenous neutrophils.44 We have modeled immunosuppression with MRSA infection in mice. These experiments showed the advantage of exogenous neutrophil transfusion. They also showed that granulocytes expressing high levels of ACE (NeuACE) appear far more effective in reducing the bacterial counts within MRSA skin lesions.
ACE inhibitors are used by millions of patients to treat hypertension, diabetic nephropathy, heart failure, and other cardiovascular diseases. Many of these patients are elderly. Although clinical studies have documented the effectiveness of renin-angiotensin system blockade, it is now possible to achieve such blockade with either an ACE inhibitor or an Ang II AT1 receptor antagonist. ACE inhibitors are generally considered safe, but some studies have noted an association between ACE inhibition and increased risk of infection. For example, studies demonstrated a higher incidence of urinary tract infections in individuals taking ACE inhibitors.45,46 A clinical study of sepsis comparing ACE inhibitors and AT1 antagonists showed that the risk of bacterial infection was increased with ACE inhibition but not with an AT1 receptor antagonist.47 These results have been challenged by other publications.48,49 Although ACE affects many physiologic systems, any reduction of neutrophilic function might be expected to contribute to an increased risk of infection; therefore, our findings have clinical implications and suggest that the link between the use of ACE inhibitors and infection should be further explored.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Makoto Katsumata (Mouse Genetics Core, Cedars Sinai Medical Center, Los Angeles, CA) for help in generating the NeuACE mice and Stacey Kolar (Division of Pediatric Infectious Diseases and Immunology, Cedars Sinai Medical Center, Los Angeles, CA) for her suggestions concerning the in vitro blood killing experiments.
This study was supported by grants from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (P01HL129941 and R01HL110353) (K.E.B.), NIH, National Institute of Allergy and Infectious Diseases (R21AI114965) (K.E.B.), and NIH, National Institute of Diabetes and Digestive and Kidney Diseases (R03DK101592) (R.A.G.-V).
Authorship
Contribution: Z.K. was responsible for study conception and experimental design, and experimental input with assistance from X.Z.S. (intellectual advice, bone marrow transplantation, and neutrophil oxidative burst assay), E.A.B. (animal husbandry and transgenic mice genotyping), S.F. (vector construction), J.F.G., M.E., and T.V.Z. (blood collection and ACE activity measurement); R.A.G.-V. contributed intellectual advice and support; G.Y.L. contributed intellectual advice, helped formulate the hypothesis, and edited the manuscript; K.E.B. contributed intellectual advice and project coordination; and Z.K. and K.E.B. analyzed data and prepared the manuscript.
Conflict-of-interest disclosure: R.A.G.-V. is an employee and a stockholder of Pfizer, Inc. The remaining authors declare no competing financial interests.
Correspondence: Kenneth E. Bernstein, Department of Pathology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; kenneth.bernstein@cshs.org.

![Figure 4. ACE increases neutrophil oxidative response. (A) ROS production in WT and NeuACE neutrophils under basal conditions and 48 hours after MRSA infection. ROS production by neutrophils was assessed by FCM analysis of DHR 123 oxidation. ROS production was significantly higher in NeuACE vs WT neutrophils. (B) ROS inhibition by DPI (3 mg/kg per day, intraperitoneal [IP]) eliminated the increased bacterial resistance of NeuACE mice. DPI injection was started 1 day before subcutaneous MRSA infection (1 × 108 CFU/mouse flank) and continued until the end of the experiment. (i) Skin lesion area, (ii) Bacterial counts (CFU) in lesions after 3 days. (C) Superoxide production was measured by SOD-inhibitable cytochrome c reduction. Neutrophils were purified from bone marrow and challenged with MRSA in vitro (n = 5/group). (D) Upon activation, the level of NOX2 subunits was measured by western blotting of neutrophil membranes. Neutrophils were purified from bone marrow and activated with MRSA for 15 minutes, and then membranes were isolated using a kit from ThermoFisher. (E) Expression of NOX2 subunits was determined by western blotting of total neutrophil protein lysate. (F) p47-phox phosphorylation (p-Ser345) was determined in neutrophil membranes by western blot analysis. Neutrophils were purified from bone marrow and activated with MRSA (MOI ∼10): (i) groups without MRSA infection (0 minutes) were used as a control, (ii) phospho-p47-phox at 5 minutes after MRSA infection, (iii) phospho-p47-phox at 15 minutes after MRSA infection, (iv) groups with ACE inhibition by ramipril. (G) Effect of gp91 ds-tat, a specific NOX2 inhibitor, on blood killing of MRSA. Blood samples were treated with gp91 ds-tat peptide for 90 minutes (5 μM), and then infected with MRSA at ∼106 CFU/mL. NOX2 inhibition eliminated the difference between WT and NeuACE bacterial killing. (D-G) Data are representative of at least 4 mice in each group. *P ≤ .05, **P ≤ .005, ***P ≤ .0005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/3/10.1182_blood-2016-11-752006/4/m_blood752006f4.jpeg?Expires=1763671464&Signature=10i2My7dsLAsLOh8BKj567jVhMYt2GxVFEoqCdVZP1EsafuYEiMBH7Tlq9G8aLeNEAbrG~34Gutm5Z2CAQgZ-veMHUNh-P1aE51MRJZqjmfKTFPnVUZCUfMN~khNW9o1QA8mQtEL4ZgSAl7SgD22f8AxLe7g40wDSO4MrF918VMQQS1XA3khjau6q-SXeaMcoBP8oSyZnH2phN5zFJZv4SCW7TsGsaTNfG6FVqQrGbqjZlXAnGgDunVwSVrERvTkF3Sect2J-WGvqMX5IUbMbT3I6XH938H5VPySdcO-mpJJyfHsfcu~2BHCpCDLw53cgg2LjJ~3ailp6FJgK2FMYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal