Key Points
TKI-resistant CML patients display persistent BMP pathway alterations in leukemic immature cells and their niche.
A subpopulation of TKI-resistant leukemic stem cells survives through binding of BMP4 to BMPR1b, which preserves TWIST-1-expressing cells.
Abstract
The BCR-ABL specific tyrosine kinase inhibitors (TKI) changed the outcome of chronic myeloid leukemia (CML), turning a life-threatening disease into a chronic illness. However, TKI are not yet curative, because most patients retain leukemic stem cells (LSC) and their progenitors in bone marrow and relapse following treatment cessation. At diagnosis, deregulation of the bone morphogenetic protein (BMP) pathway is involved in LSC and progenitor expansion. Here, we report that BMP pathway alterations persist in TKI-resistant patients. In comparison with patients in complete cytogenetic remission, TKI-resistant LSC and progenitors display high levels of BMPR1b expression and alterations of its cellular localization. In vitro treatment of immature chronic phase CML cells with TKI alone, or in combination with interferon-α, results in the preferential survival of BMPR1b+ cells. We demonstrated persistent and increasing BMP4 production by patients’ mesenchymal cells with resistance. Patient follow-up revealed an increase of BMPR1b expression and in BMP4 expression in LSC from TKI-resistant patients in comparison with diagnosis, while remaining unchanged in sensitive patients. Both leukemic and nonleukemic cells exhibit higher BMP4 levels in the bone marrow of TKI-resistant patients. Exposure to BMP2/BMP4 does not alter BCR-ABL transcript expression but is accompanied by the overexpression of TWIST-1, a transcription factor highly expressed in resistant LSC. By modulating BMP4 or BMPR1b expression, we show that these elements are involved in TKI resistance. In summary, we reveal that persistence of BMP alterations and existence of an autocrine loop promote CML-primitive cells’ TKI resistance.
Introduction
Tumor heterogeneity might arise from cancer stem cells (SC) that would confer the ability to proliferate extensively and regenerate upon treatment cessation or acquired resistance. This heterogeneity that also concerns leukemic SC (LSC) has recently been substantiated by transcriptomic analysis.1,2 These single-cell approaches linked specific subpopulations to a primitive quiescent phenotype that persisted throughout therapy. Eradication of this subpopulation of cells represents a mandatory step in any clinical setting for cure. A unique and outstanding model for addressing such questions is chronic myelogenous leukemia (CML), arising from the transformation of a SC by the BCR-ABL oncogene. Without treatment, this disease evolves into an inexorable fatal blast crisis. In addition to allogeneic SC transplantation, interferon-α (IFN-α) induced stable remissions in some chronic phase (CP) CML patients,3,4 which correlated with long-term survival,5,6 but was responsible for side effects. Tyrosine kinase inhibitors (TKI), such as imatinib mesylate (IM), are targeted therapies specifically inhibiting the BCR-ABL kinase activity. These agents revolutionized CML patient’s medical care and now represent frontline treatment. However, despite their efficacy, approximately 30% of patients will not respond optimally and, in some cases, resistance mechanisms remain unclear.7 TKI fail to eliminate LSC persisting in patients in complete cytogenetic remission (CCyR)8,9 as shown by the indefinite detection of BCR-ABL+ residual cells in their bone marrow (BM).2 These cells do not depend on the BCR-ABL activity for their survival as TKI insensitive10,11 and are likely responsible for the high rate of relapse, even in patients with a durable undetectable disease after treatment discontinuation.12,13 Studies assessing the potential of combination therapies aimed at either directly killing LSC or forcing them out of their “stem” protective state have yet to provide convincing evidence of success.14-16 It suggests that resistance could emerge from the heterogeneity of LSC subpopulations depending on the cues offered by their microenvironment. We, and others, have identified deregulations of the bone morphogenetic protein (BMP) signaling in LSC and their microenvironment (niche) in CML patients.17-19 These molecules are involved in SC regulation.20 Among the BMPs, BMP4 is crucial in allowing mesodermal embryonic cell commitment to the hematopoietic lineage.21 In human cord blood and adults, BMP4 regulates SCs and their niche22-24 such as progenitor differentiation into megakaryocytes,25 whereas BMP2 favors erythropoietic commitment.26
Deregulation of the BMP pathway at the intrinsic and extrinsic levels has been reported in hematological malignancies19,27 and is involved in the cancer SC phenotype in solid tumors,17,28-31 suggesting that it represents a general feature of transformed SC events that could contribute to tumor heterogeneity. In CP-CML patients at diagnosis, the BMP pathway is deregulated with soluble BMPs, secreted in the tumor niche that maintains a fraction of BMPR1b+ LSCs and amplifies leukemic progenitors, participating in disease progression.17 The basic helix-loop-helix (bHLH) transcription factor TWIST-1 is overexpressed in immature compartments and represents a predictive factor of treatment resistance of CML.32-34 TWIST-1 overexpression promotes cell growth, tumor-initiating properties, and drug resistance and increases clonogenic capacities in other myeloid leukemias.33 Interestingly, TWIST-1 can be a regulator or a target of the BMP pathway in different cell models.35,36 Here, we analyzed BMP pathway alterations in leukemic cells from TKI-treated CML patients in TKI resistance or molecular progression and investigated BMP ability to induce LSC survival in vitro.
Methods
Cells
Samples were obtained from healthy BM donors for allogeneic transplant or from peripheral blood (PB) or BM from CML patients in CP at diagnosis before TKI treatment or after receiving TKI treatment (for patients who achieved CCyR, only BM samples were used). All donors provided written informed consent in accordance with the Declaration of Helsinki. Studies were approved by local ethics committee bylaws. Definitions of response and resistance respected the last European LeukemiaNet recommendations.37 CD34 immunomagnetic separation (StemCell Technologies, Vancouver, BC, Canada) reached an average purity of 90%. Mesenchymal stem cells (MSCs) were isolated from BM samples and cultivated as described.17 KCL22-sensitive (KCL22S) and KCL22-resistant (KCL22R) cell lines, obtained from F. X. Mahon (Institut Bergonié, Bordeaux, France),38 were cultured in RPMI1640 10% fetal calf serum media. Cells were transfected using the Neon Transfection System (Invitrogen) by 1 µg of plasmid per 5 × 105 cells of pCAG-ires, pCAG-ires-BMPR1b, hPGK-empty, or hPGK-BMP4 plasmid for KCL22S cells and of pX2sh-control or pX2sh-BMPR1B for KCL22R cells.32 Transfected cells were then selected with Puromycin (Invitrogen).
Functional assays
Colony forming cell (CFC) and long-term culture-initiating cell (LTC-IC) assays were performed as reported.17,25 Fresh CD34+ cells from newly diagnosed CP (CP-Diag) patients were harvested and cultured for 5 days in serum-free Iscove modified Dulbecco medium (Invitrogen), 15% bovine serum albumin, insulin, and transferrin (BIT) (StemCell Technologies), supplemented with 10 ng/mL of interleukin-3 (IL-3) and IL-6 and 50 ng/mL of stem cell factor and FLT3-L (Partech, Neuilly-sur-Seine, France). IM was added at 1 or 2 µM (Novartis Pharma, Basel, Switzerland) in the presence or not of interferon-α2a (1000 U; Roche, Basel, Switzerland); BMP2 and BMP4 (10 ng/mL); or sBMPR1b (4 µg/mL) (R&D Systems). Viable cells were counted by trypan blue exclusion.
Flow cytometry
Staining of cells was performed using Cya5-phycoerythrin (PE)–conjugated anti-CD34, PE-conjugated anti-CD38 or fluorescein isothiocyanate–conjugated anti-CD38 (BD Biosciences) and PE-conjugated anti-BMPR1b (R&D Systems).17
RNA analysis
Following lysis in Tri Reagent (Sigma-Aldrich), quantitative reverse transcription was performed using a Lighcycler-480 II (RocheLife Science); the described protocol, reagents, and primers are listed in our previous work.17,32 The expression level for each gene was normalized on CD34− cells isolated from normal donor cells and used as a reference sample.
BMP quantification
BM plasma were precleared of cellular debris by rapid centrifugation and processed for BMP2 and BMP4 enzyme-linked immunosorbent assay (ELISA) quantification (R&D Systems).17 Western blot was performed using monoclonal antibodies to TWIST-1, BMPR1b, and BMP4 (Abcam, Cambridge, United Kingdom) or glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling).26
Statistics
Mean comparisons were performed with a bilateral Mann-Whitney test (paired or unpaired as required), and correlations were determined with a Pearson bilateral test, using GraphPad Prism software (La Jolla, CA). We performed a matrix correlation between a set of variables using a Spearman nonparametric bilateral test (GraphPad Prism), with a confidence interval of 95%. Data represent the coefficient of correlation ρ, ranging from −1 (maximal negative) to +1 (maximal positive), illustrated by a scale of colors (R software, Corrplot package). Significant P values are indicated by asterisks in the figures.
Results
BMPR1b remained highly expressed in TKI-resistant CML patients
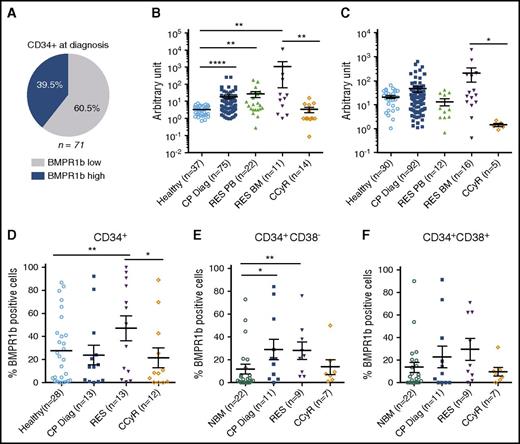
At diagnosis, we reported a higher expression of BMPR1b in immature CP-CML cells involved in the maintenance and expansion of LSC and leukemic progenitors in response to an exogenous BMP2/BMP4 signal.17 Patients displaying high levels of BMPR1b transcripts represent approximately 40% of newly diagnosed CP-CML patients (Figure 1A). We then investigated whether high levels of BMPR1b return to baseline following TKI treatment. We confirmed on a larger cohort the higher-transcript expression of BMPR1b at CP-Diag in both mononuclear cells (MNC) (Figure 1B) and CD34+ cells (Figure 1C) and by analyzing a large- screen CML published dataset39 (supplemental Figure 1A, available on the Blood Web site). These data from literature, together with our own analysis of cells isolated from patients at various stages of CML (supplemental Figure 1B), suggest that this difference is not predictive of disease progression. Following TKI treatment, we measured higher BMPR1b transcript levels in MNCs (p = .0025) and in CD34+ (p = .0108) BM cells isolated from TKI-resistant (RES) patients (cytogenetic or molecular progression, or both)37 in comparison with those in remission (CCyR) (Figure 1B-C). Unlike circulating (PB) mature MNCs, BMPR1b levels in circulating CD34+ isolated from TKI-resistant patients remained similar to healthy cell levels (Figure 1B-C), suggesting that the subfraction of BMPR1b-high immature cells remained in their BM leukemic niche. The same pattern was observed by flow cytometry in CD34+ BM cells that displayed higher cell membrane expression of BMPR1b in TKI-resistant samples in comparison with CCyR samples (Figure 1D). Using triple staining, we identified BMPR1b+-BM cells in the stem (CD34+CD38−) and progenitor (CD34+CD38+) compartments at diagnosis, at CCyR or at TKI resistance. In one illustrative example we observed a higher expression of BMPR1b in CD34+CD38− and CD34+CD38+ cells at diagnosis, unlike those in control healthy BM donors (supplemental Figure 2C, upper panels). Conversely to CCyR-CD34+CD38− cells, BMPR1b remained highly present at the membrane of TKI-resistant immature cells (supplemental Figure 2C, lower panels). Although not always significant, the proportion of cells expressing BMPR1b at their membrane was increased in both SC (Figure 1E) and progenitor (Figure 1F) compartments in resistant patients in comparison with TKI responders. These data suggest that BMPR1b-expressing cells specifically survive in TKI-unresponsive patients.
TKI-resistant BM immature cells express higher levels of BMP1b receptors. (A) Frequency of BMPR1blo or BMPR1bhi patients in CML patients at diagnosis defined as previously described,17 and presented here in a pie chart. Comparative transcriptional expression of BMPR1b genes in BM cells from healthy donors, or CML patients in chronic phase at diagnosis (CP-Diag), resistant (RES) to TKI or in remission (CCyR) in MNC (B) or CD34+ selected cells (C). Cell membrane analysis of BMPR1b was performed by flow cytometry, and data represent the percentage of positive cells at cell surface in CD34+ (D) or CD34+CD38− (E) stem cells and CD34+CD38+ (F) progenitor compartment in BM samples obtained from healthy donors, or CML patients in chronic phase at diagnosis, resistant to TKI treatment or in remission. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
TKI-resistant BM immature cells express higher levels of BMP1b receptors. (A) Frequency of BMPR1blo or BMPR1bhi patients in CML patients at diagnosis defined as previously described,17 and presented here in a pie chart. Comparative transcriptional expression of BMPR1b genes in BM cells from healthy donors, or CML patients in chronic phase at diagnosis (CP-Diag), resistant (RES) to TKI or in remission (CCyR) in MNC (B) or CD34+ selected cells (C). Cell membrane analysis of BMPR1b was performed by flow cytometry, and data represent the percentage of positive cells at cell surface in CD34+ (D) or CD34+CD38− (E) stem cells and CD34+CD38+ (F) progenitor compartment in BM samples obtained from healthy donors, or CML patients in chronic phase at diagnosis, resistant to TKI treatment or in remission. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
BMPR1b-high immature cells survive TKI-treatment better than do BMPR1b-low cells
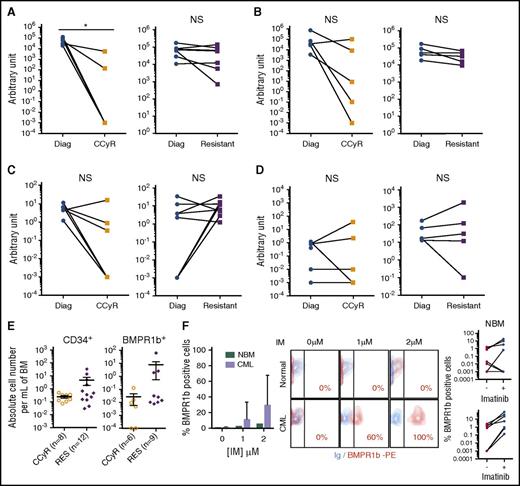
Our data suggest that a subpopulation of leukemic BMPR1b+ cells remain present in the BM of patients who do not respond to TKI while being eliminated in responding patients. To test this hypothesis, we followed the expression of BMPR1b in the same patients at diagnosis and after TKI treatment. In the majority of cases, BMPR1b-high cells were efficiently targeted after 3 months of TKI treatment, as illustrated by their almost complete eradication in comparison with that at diagnosis (supplemental Figure 1D). This was confirmed at transcript levels in MNC or CD34+ BM cells from CCyR patients or at treatment failure or relapse. In comparison with values measured at diagnosis (Diag), TKI-treatment efficiency was indicated by the decrease in BCR-ABL levels in MNC (Figure 2A) or CD34+ cells (Figure 2B) of responding patients (CCyR), however significant (P < .01), only in the most mature compartment. As was expected, BCR-ABL level was not significantly affected in both cell types of nonresponding patients (RES). The level of BMPR1b decreased in more than 80% of MNCs from TKI-responsive patients (Figure 2C), whereas the impact of treatment in BM-CD34+ cells was not homogenous (Figure 2D). Levels of BMPR1b remained high in MNC and CD34+ cells of resistant patients. Unlike those in TKI-responsive patients, total numbers of BM CD34+ and BMPR1b+ cells were maintained or expanded in TKI-resistant patients (Figure 2E). To evaluate whether BMPR1b expression was affected by TKI exposure, we compared its membrane expression in CD34+ cells after 5 days of IM. BMPR1b expression was not modified in healthy cells but increased in CML CD34+ cells from 0% (without IM) to 10% and 30% after 1 and 2 µM of IM treatment, respectively (Figure 2F). In a remarkable example, this increase could reach a maximum BMPR1b expression as presented. These data indicate that TKI increases in vitro BMPR1b+CD34+ cells in CP-CML but not in normal counterparts and supports the theory that a selection of BMPR1b+ subpopulation of immature cells could occur in vivo under TKI pressure. This set of data supports the hypothesis that TKI resistance is accompanied by the persistence of BMPR1bhi cells within the immature compartment.
TKI treatment result in BMPR1b-positive CD34+cell accumulation in resistant patients. Follow-up of transcriptional expression of BCR-ABL (A-B) and BMPR1b (C-D) genes in BM cells from CML patients at diagnosis and in remission or resistant under TKI in MNC (A-C) or CD34+ (B-D) selected cells. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent the mean values ± SEM of the indicated number of analyzed samples. (E) Absolute number of cells of CD34 or BMPR1b positive cells per milliliter of BM in TKI-resistant patients and in CCyR patients presented as individual value per patient. The heavy horizontal lines represent the mean values ± SEM of the indicated number of analyzed samples. (F) Experimental protocol: CD34+ cells from newly diagnosed CP patients treated or not in serum-free media with IM at 1 or 2 µM. After 5 days of culture, cells were analyzed for BMPR1b expression by flow cytometry. Data are presented as a graph that represents the percentage of mean value of BMPR1b positive cells ± SEM of five independent samples (left panel), illustrated by one representative dot plot histogram and a representation of individual values for each sample treated or not with imatinib (mean of values obtained for cells treated with 1 or 2 μM of IM) (right panel). NBM, normal bone marrow. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
TKI treatment result in BMPR1b-positive CD34+cell accumulation in resistant patients. Follow-up of transcriptional expression of BCR-ABL (A-B) and BMPR1b (C-D) genes in BM cells from CML patients at diagnosis and in remission or resistant under TKI in MNC (A-C) or CD34+ (B-D) selected cells. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent the mean values ± SEM of the indicated number of analyzed samples. (E) Absolute number of cells of CD34 or BMPR1b positive cells per milliliter of BM in TKI-resistant patients and in CCyR patients presented as individual value per patient. The heavy horizontal lines represent the mean values ± SEM of the indicated number of analyzed samples. (F) Experimental protocol: CD34+ cells from newly diagnosed CP patients treated or not in serum-free media with IM at 1 or 2 µM. After 5 days of culture, cells were analyzed for BMPR1b expression by flow cytometry. Data are presented as a graph that represents the percentage of mean value of BMPR1b positive cells ± SEM of five independent samples (left panel), illustrated by one representative dot plot histogram and a representation of individual values for each sample treated or not with imatinib (mean of values obtained for cells treated with 1 or 2 μM of IM) (right panel). NBM, normal bone marrow. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Ligands binding to BMPR1b mediate LSC survival to TKI treatment
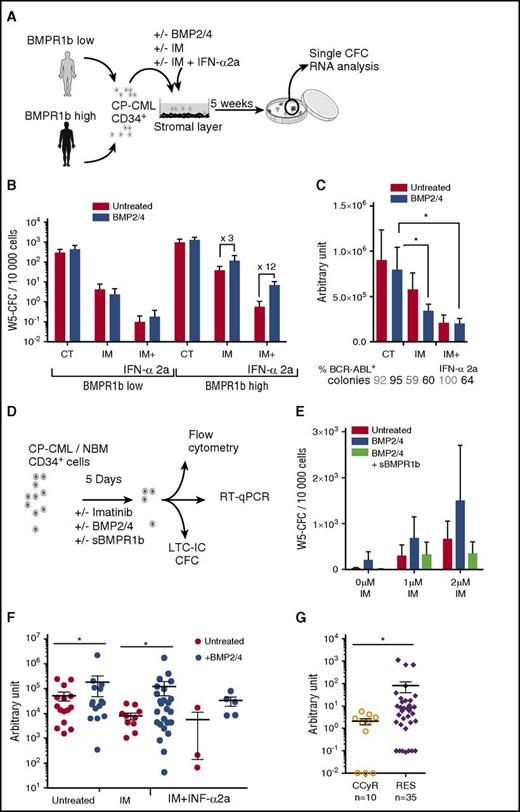
To investigate whether BMPR1b+ immature cells are involved in TKI resistance, we used cells isolated from CP-Diag CML samples and divided them into two groups according to their BMPR1b transcript level, as described.17 To evaluate LSC survival to treatment, we cultured sorted CD34+ cells in LTC-IC assays, with or without IM, alone or in combination with IFN-α2a, and in the presence or not of BMP2/BMP4 (Figure 3A). Cells from the BMPR1bhi group contained approximately threefold more LTC-IC (revealed by stem-cell-derived progenitors quantified by CFC assay following long-term culture: W5-CFC) than did BMPR1blo samples and expanded more in the presence of BMP2/BMP4, though this was not observed for progenitors (CFC) (supplemental Figure 2A-C). Cells from the BMPR1bhi group survive more than do cells from the BMPR1blo group, when treated with IM alone (10 times) and with IFN-α2a (7 times) (Figure 3B). BMP2/BMP4 strongly increased W5-CFC in BMPR1bhi samples despite treatments by IM alone (threefold) or with IFN-α2a (12-fold). Conversely, combination of IM+IFN-α2a almost fully eradicated LTC-IC in BMPR1blo samples, and the addition of BMP2/BMP4 did not change the output. Individual LTC-IC-derived colonies from BMPR1bhi samples were in majority BCR-ABL+ (Figure 3C), ensuring that a remaining Ph− LTC-IC subfraction was not amplified. Efficacy of treatment was confirmed by the significant decrease in BCR-ABL transcript levels and unaffected by BMP2/BMP4 addition (Figure 3C). Sorted CP-Diag CD34+ CML cells were pretreated 5 days in serum-free conditions with IM alone or in combination with BMP2/BMP4 and in the presence, or not, of the soluble BMPR1b (sBMPR1b), the only available type 1b–specific inhibitor (Figure 3D).17,40 The use of sBMPR1b fully reverts BMP2/BMP4-mediated LTC-IC expansion, confirming the importance of ligand binding to BMPR1b for the survival of LSC (W5-CFC output) (Figure 3E), while leukemic progenitors remain unaffected (supplemental Figure 2D).
BMPR1b+CD34+cell survival to TKI is promoted by exogenous treatment with BMP2/4. (A) Experimental protocol for LTC-IC assays performed using CD34+ cells from BMPR1blo or BMPR1bhi newly diagnosed CP patients. Medium was supplemented weekly with IM (1 µM), or IM (1 µM) and INF-α2a (1,000 U) (IM + IFN-α2a), with or without BMP2 and BMP4 (10 ng/mL each). (B) LTC-IC output is presented as mean ± SEM of the number of colonies for 10 000 cells of week 5 CFC for BMPR1blo (n = 6) or BMPR1bhi (n = 6) patients. (C) BCR-ABL gene expression was analyzed in individual colonies from week 5 CFC obtained from the BMPR1bhi patient subgroup. Percentage of BCR-ABL-expressing colonies is indicated at the bottom of the chart. The figure represents the mean ± SEM of BCR-ABL expression in BCR-ABL-positive colonies. (D) Experimental protocol: CD34+ cells from newly diagnosed CP patients treated or not in serum-free media with IM at 1 or 2 µM, and in the presence or not of BMP2, BMP4, and with or without sBMPR1b. After 5 days of culture, cells were harvested and analyzed for BMPR1b expression by flow cytometry, for genes expression by quantitative polymerase chain reaction and at functional level by CFC and LTC-IC assays. (E) LTC-IC output presented as mean ± SEM of week 5–derived CFC colonies for 10 000 input cells. (F) TWIST-1 gene expression analyzed in individual colonies from week 5 CFC obtained from BMPR1bhi patient subgroups that survived IM treatment alone or in the presence of INF-α2a (panel A). Data from individual colonies are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM. (G) Comparative transcriptional expression of TWIST-1 gene in PB or BM CD34+ selected cells from CCyR patients and TKI-resistant patients. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. W5, week 5. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
BMPR1b+CD34+cell survival to TKI is promoted by exogenous treatment with BMP2/4. (A) Experimental protocol for LTC-IC assays performed using CD34+ cells from BMPR1blo or BMPR1bhi newly diagnosed CP patients. Medium was supplemented weekly with IM (1 µM), or IM (1 µM) and INF-α2a (1,000 U) (IM + IFN-α2a), with or without BMP2 and BMP4 (10 ng/mL each). (B) LTC-IC output is presented as mean ± SEM of the number of colonies for 10 000 cells of week 5 CFC for BMPR1blo (n = 6) or BMPR1bhi (n = 6) patients. (C) BCR-ABL gene expression was analyzed in individual colonies from week 5 CFC obtained from the BMPR1bhi patient subgroup. Percentage of BCR-ABL-expressing colonies is indicated at the bottom of the chart. The figure represents the mean ± SEM of BCR-ABL expression in BCR-ABL-positive colonies. (D) Experimental protocol: CD34+ cells from newly diagnosed CP patients treated or not in serum-free media with IM at 1 or 2 µM, and in the presence or not of BMP2, BMP4, and with or without sBMPR1b. After 5 days of culture, cells were harvested and analyzed for BMPR1b expression by flow cytometry, for genes expression by quantitative polymerase chain reaction and at functional level by CFC and LTC-IC assays. (E) LTC-IC output presented as mean ± SEM of week 5–derived CFC colonies for 10 000 input cells. (F) TWIST-1 gene expression analyzed in individual colonies from week 5 CFC obtained from BMPR1bhi patient subgroups that survived IM treatment alone or in the presence of INF-α2a (panel A). Data from individual colonies are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM. (G) Comparative transcriptional expression of TWIST-1 gene in PB or BM CD34+ selected cells from CCyR patients and TKI-resistant patients. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. W5, week 5. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Considering that TWIST-1 overexpression is involved in TKI resistance of CD34+-CML cells,32 we assessed its expression in BMPR1bhi W5-CFC and observed a decrease of its expression in IM conditions, amplified by IFN-α2a (Figure 3F). In comparison with control, BMP2/BMP4-treated cells displayed a fourfold increase in W5-CFC. BMP2/BMP4 increased TWIST-1 transcripts even in IM (13-fold) or IM+IFN-α2a (twofold) surviving leukemic colonies. These results suggest that BMPR1b+ LSC are resistant to IM through a BMP-mediated TWIST-1 upregulation. This is also supported by higher levels of TWIST-1 detected in CD34+ cells of the majority of TKI-resistant patients (Figure 3G). These results demonstrate that functionally defined primitive BMPR1bhi cells from CP-CML patients remain less sensitive to IM, even when combined with IFN-α2a, likely through high levels of TWIST-1.
TKI-resistant CML leukemic and nonleukemic cells produce high levels of BMP4
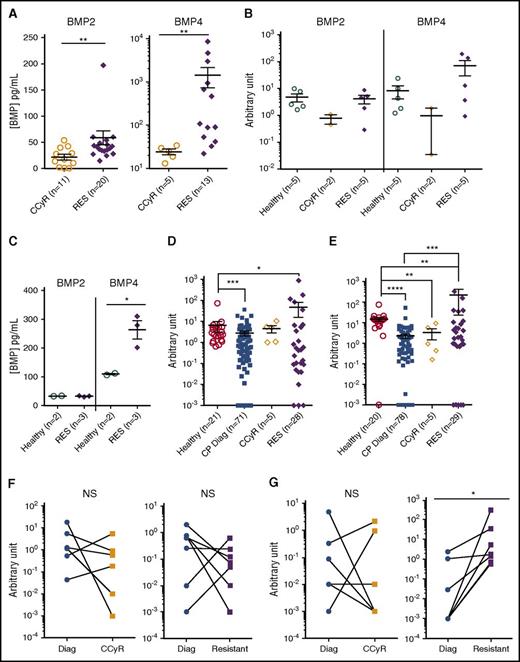
At diagnosis, we reported higher concentrations in the BM plasma of BMP2 and BMP4 of CP-CML patients than in that of healthy donors. These proteins are abnormally produced by cells of the microenvironment, such as MSCs.17 Here, we have observed significantly higher levels of soluble BMP2 and BMP4, in the BM plasma of TKI-resistant patients in comparison with CCyR patients (Figure 4A). Lower BMP2 transcript levels were detected in CCyR-derived MSC from patients under TKI treatment, whereas no difference with healthy MSC was observed for TKI-resistant derived MSC (Figure 4B). Conversely, higher levels of BMP4 were observed in MSC derived from resistant patients in comparison with healthy or CCyR samples. This was confirmed at the protein level by measuring soluble BMP2 and BMP4 synthesized de novo by MSC cultured 48 h in serum-free medium. MSC-conditioned media indicated that cells derived from resistant patients produce significantly higher amounts of BMP4 than do healthy cells, whereas no difference was observed for BMP2 (Figure 4C). When we analyzed BMP2 or BMP4 transcript levels in CD34+ cells isolated from CP-CML at diagnosis or from TKI-treated patients achieving or not achieving CCyR, a decrease or no difference was observed at diagnosis or in patients achieving CCyR, respectively, in comparison with healthy BM cells (Figure 4D-E). Conversely, CD34+ cells from TKI-resistant patients expressed higher levels of BMP2 and, even more significantly, of BMP4 transcripts (Figure 4D-E, supplemental Figure 2E). Patient follow-up since diagnosis indicated no reproducible and overall significant difference between diagnosis and after TKI treatment of BMP2 transcript levels in both CCyR patients and nonresponders (resistant) (Figure 4F). Conversely, a significant increase of BMP4 expression (P < .01) is measured in all patient samples at resistance, whereas no treatment effect is observed in CCyR samples (Figure 4G). These data indicate that unlike at diagnosis,17 an autocrine production of BMP4 takes place in CD34+ TKI-resistant CML cells. Our results show that BMP4 is produced not only by MSC of the leukemic microenvironment but also by LSC themselves.
Higher-ligand expression in TKI-resistant BM microenvironment linked to leukemic and nonleukemic BMP2 and BMP4 production. (A) ELISA quantification of BMP2 and BMP4 in BM plasma obtained from CCyR patients and resistant patients. Results from individual samples are expressed in picograms per milliliter, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. (B-C) MSCs were isolated, as has been described17 from BM samples of healthy donor or CP-CML patients at remission or who were TKI resistant and analyzed after one passage in culture for their transcriptional expression (B) or de novo production (C) of soluble of BMP2 or BMP4 after 72 h of culture in serum-free media. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. Transcriptional expression of BMP2 (D) or BMP4 (E) in CD34+ cells isolated from healthy BM or CP-CML samples of patients at diagnosis, in remission or from TKI-resistant patients. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. Follow-up of transcriptional expression of BMP2 (F) and BMP4 (G) genes in BM cells from CML patients at diagnosis and when they achieved remission or were declared resistant to TKI treatment in CD34+ selected cells. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Higher-ligand expression in TKI-resistant BM microenvironment linked to leukemic and nonleukemic BMP2 and BMP4 production. (A) ELISA quantification of BMP2 and BMP4 in BM plasma obtained from CCyR patients and resistant patients. Results from individual samples are expressed in picograms per milliliter, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. (B-C) MSCs were isolated, as has been described17 from BM samples of healthy donor or CP-CML patients at remission or who were TKI resistant and analyzed after one passage in culture for their transcriptional expression (B) or de novo production (C) of soluble of BMP2 or BMP4 after 72 h of culture in serum-free media. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. Transcriptional expression of BMP2 (D) or BMP4 (E) in CD34+ cells isolated from healthy BM or CP-CML samples of patients at diagnosis, in remission or from TKI-resistant patients. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. Follow-up of transcriptional expression of BMP2 (F) and BMP4 (G) genes in BM cells from CML patients at diagnosis and when they achieved remission or were declared resistant to TKI treatment in CD34+ selected cells. Results from individual samples analyzed are expressed in arbitrary units, and heavy horizontal lines represent mean values ± SEM of the indicated number of analyzed samples. NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Increase of BMP4, BMPR1b, and TWIST-1 expression in LSC upon resistance in a demonstrative patient
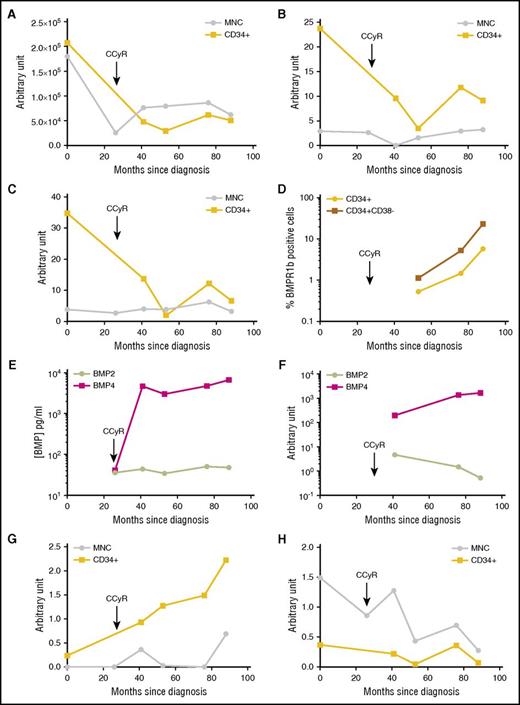
We followed the expression of BMPR1b, BMP2, BMP4, and TWIST-1 during disease evolution of a single patient over 7 years. BCR-ABL expression rapidly decreased following frontline dasatinib treatment in both MNC and CD34+ cells as measured at diagnosis (month 0) and after long-term CCyR (26 months later) (Figure 5A). At relapse, the BCR-ABL transcript level increased and stabilized at high levels as detected 40 months postdiagnosis and later. As has been reported,32 TWIST-1 expression was higher within CD34+ cells and followed the same expression pattern as that of BCR-ABL, however delayed (Figure 5B). The BMPR1b follow-up was very similar, with a higher expression in CD34+ cells at relapse after an initial decrease following treatment (Figure 5C). The proportion of immature CD34+ and LSC-CD34+CD38− cells that express BMPR1b at the membrane level progressively increases with time (Figure 5D), suggesting that although transcript levels increase could be modest, BMPR1b expression/localization at the cell membrane could change upon resistance. This is indeed observed in the CML model of KCL22 cells with a detection of the BMPR1b at the cell surface only in the KCL22-resistant (KCL22R) cell line38 while undetected in its TKI-sensitive counterpart (KCL22S), and there are no changes in the cytoplasmic expression of this receptor (supplemental Figure 3A). The BM environment content of soluble BMP2 and BMP4 changed during disease evolution, as detected by ELISA at remission of this patient and later on (Figure 5E). It confirmed previous data (Figure 4A) with a predominant accumulation of BMP4 over time. Unlike that for BMP2 (Figure 5F,H), BMP4 transcript level increases in MSCs (Figure 5F) and CD34+ leukemic cells (Figure 5G) of the patient. This is in line with data obtained for unrelated patients (Figure 4C-E) or during short-term follow-up of patients (Figure 4F-G). This suggests that the intrinsic expression of BMP4 by leukemic cells could reflect a disease evolution toward more aggressive stages. This hypothesis is sustained by analyzing BMP4 expression in a published dataset (supplemental Figure 2B).39 Data obtained from the long-term follow-up of this patient strongly suggest a close relationship between deregulation of BMP4, its BMPR1b receptor, and resistance of immature leukemic cells characterized by BCR-ABL and TWIST-1 expressions.
Long-term follow-up of a demonstrative patient who became refractory to all TKI treatments. Follow-up for 88 months of a newly diagnosed CP-CML patient. Since diagnosis, this patient had no detectable BCR-ABL mutation, had an intermediate Sokal, and received dasatinib as first-line therapy. BM and PB samples were harvested after 26 months of treatment (still in CCyR) then upon resistance after 41, 53, 76, and 88 months after diagnosis. Follow-up of transcriptional expression was performed for BCR-ABL (A), TWIST-1 (B), and BMPR1b (C) genes in MNC- or CD34+-selected cells, as indicated on the graphs. Results from each analyzed sample are expressed in arbitrary units. (D) Cell membrane expression of BMPR1b was analyzed by flow cytometry on CD34+ or CD34+CD38− selected cells. (E) ELISA quantification of BMP2 and BMP4 in BM plasma at the indicated time of harvest. Results from each sample are expressed in picograms per milliliter. (F) Follow-up of transcriptional expression was performed for BMP2 and BMP4 genes in mesenchymal stem cells isolated from BM harvested after 53, 76, and 88 months after diagnosis. Follow-up of transcriptional expression was performed for BMP4 (G) and BMP2 (H) genes in MNC- or CD34+-selected cells, as indicated on the graphs.
Long-term follow-up of a demonstrative patient who became refractory to all TKI treatments. Follow-up for 88 months of a newly diagnosed CP-CML patient. Since diagnosis, this patient had no detectable BCR-ABL mutation, had an intermediate Sokal, and received dasatinib as first-line therapy. BM and PB samples were harvested after 26 months of treatment (still in CCyR) then upon resistance after 41, 53, 76, and 88 months after diagnosis. Follow-up of transcriptional expression was performed for BCR-ABL (A), TWIST-1 (B), and BMPR1b (C) genes in MNC- or CD34+-selected cells, as indicated on the graphs. Results from each analyzed sample are expressed in arbitrary units. (D) Cell membrane expression of BMPR1b was analyzed by flow cytometry on CD34+ or CD34+CD38− selected cells. (E) ELISA quantification of BMP2 and BMP4 in BM plasma at the indicated time of harvest. Results from each sample are expressed in picograms per milliliter. (F) Follow-up of transcriptional expression was performed for BMP2 and BMP4 genes in mesenchymal stem cells isolated from BM harvested after 53, 76, and 88 months after diagnosis. Follow-up of transcriptional expression was performed for BMP4 (G) and BMP2 (H) genes in MNC- or CD34+-selected cells, as indicated on the graphs.
BMP4, BMPR1b, and TWIST-1 expression contribute to TKI resistance
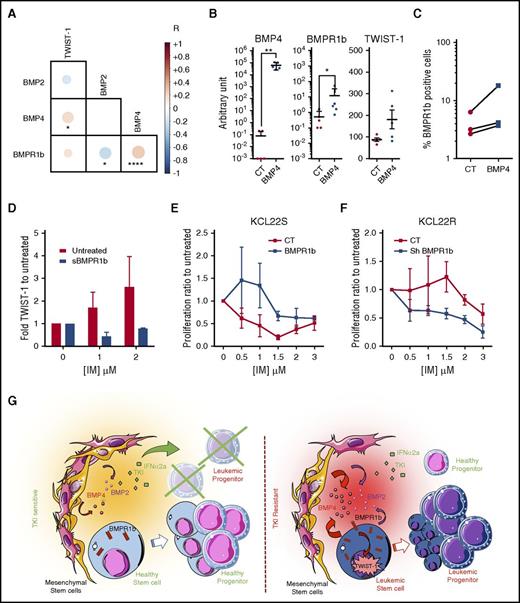
To identify and evaluate the strength and direction of association between a set of BMP pathway genes, we performed a matrix correlation using Spearman nonparametric analysis on transcriptional data from 140 independent CD34+ samples obtained from all kinds of patients, under treatment or not, at all disease phases, as described in the Methods section. Data indicated a strong link and a positive correlation among BMP4, BMPR1b, and TWIST-1 expression (Figure 6A). We observed a negative correlation between BMP2 and BMPR1b, confirming the predominance of BMP4 and BMPR1b in the context of TKI resistance. Using a TF1 CP-CML model,17 we observed that only BCR-ABL-expressing cells change their TWIST-1 transcript levels after long-term exposure to BMP2/BMP4 (supplemental Figure 2C). Conversely, we also confirmed that lengthy exposure to BMP2/BMP4 did not significantly modify BCR-ABL expression (supplemental Figure 2D). To establish a direct link between these different elements, we modulated BMP4 and BMPR1b expression in the KCL22 cell line, because this model reproduces the alterations observed in CML patient samples (supplemental Figure 3E). Endogenous induction of BMP4 expression in sensitive KCL22S results in the induction of BMPR1b expression detected at transcript and cell membrane levels (Figure 6B-C), together with an increase in the expression of TWIST-1 (Figure 6B, supplemental Figure 3F). Variability in transcript levels measured in different experiments seemed to be related to the distance with the BMP4 signal initiation, the first event being BMP4 binding to its BMPR1b receptor and a more distant event being its downstream target gene TWIST-1. (Figure 6B). These results suggest control of TWIST-1 transcription factor downstream of the BMP4 interaction with BMPR1b. It indicates that BMP4 binding to BMPR1b might prevent LSC from responding to TKI, thus favoring high transcripts levels of the resistant specific gene TWIST-1. This was further sustained when we monitored the transcript levels of TWIST-1 in the surviving CFC-derived LTC-IC colonies (Figure 3D) and observed the inhibition by sBMPR1b addition of the dose-dependent increase of TWIST-1 levels following IM treatment (Figure 6D). Lastly, inducing BMPR1b in KCL22S increases their survival after IM treatment (Figure 6E). Conversely, inhibition of BMPR1b by short hairpin RNA restores approximately 50% of KCL22R cell sensitivity to IM (Figure 6F). These data show that BMP pathway alterations persist in TKI-exposed LSC and contribute to CP-CML cell resistance, likely by controlling TWIST-1 gene expression and activating a BMP4/BMPR1b loop.
Evidence for a direct link between BMP4, BMPR1b, and TWIST-1 expression and BCR-ABL cell resistance to IM. (A) Correlation matrix representing bilateral Pearson correlation tests performed on transcriptional expression of TWIST-1, BMP2, BMP4, and BMPR1b in CD34+ BM or PB cells from 140 patients (under TKI treatment or CP-Diag CML patients). Positive correlations are presented in red, and negative ones in blue. (B) KCL22S overexpressing BMP4 or KCL22S transfected with an empty vector were analyzed for BMPR1b and TWIST-1 transcript. Results from each analyzed sample are expressed in arbitrary units. (C) Flow cytometry detection of BMPR1b at the cell surface of KCL22S overexpressing BMP4 or transfected with an empty vector. Data represent the percentage of positive cells of paired analyzed samples of control or BMP4-transfected TKI-sensitive KCL22. (D) CD34+ cells from newly diagnosed CP patients treated or not in serum-free media with IM at 1 or 2 µM, and with or without sBMPR1b. After 5 days of culture, TWIST-1 gene expression was analyzed. BMPR1b expression was modulated in the KCL22 cell line by transfection with an empty vector as a control or a BMPR1b-expressing sequence to induce its expression in KCL22-sensitive cells (E) or using a vector containing short hairpine (sh) sequence against BMPR1b to decrease its level in KCL22-resistant cells (F). After puromycin selection, cells were incubated with 1% fetal calf serum in the presence of IM at the indicated dose. After 3 days of culture, cell proliferation and viability were determined by trypan blue staining. The percentage of proliferation was determined by reference to the untreated number of viable cells ± SEM from four experiments. (G) Model for BMPR1b-mediated TKI resistance of CML LSC. CT, control; NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Evidence for a direct link between BMP4, BMPR1b, and TWIST-1 expression and BCR-ABL cell resistance to IM. (A) Correlation matrix representing bilateral Pearson correlation tests performed on transcriptional expression of TWIST-1, BMP2, BMP4, and BMPR1b in CD34+ BM or PB cells from 140 patients (under TKI treatment or CP-Diag CML patients). Positive correlations are presented in red, and negative ones in blue. (B) KCL22S overexpressing BMP4 or KCL22S transfected with an empty vector were analyzed for BMPR1b and TWIST-1 transcript. Results from each analyzed sample are expressed in arbitrary units. (C) Flow cytometry detection of BMPR1b at the cell surface of KCL22S overexpressing BMP4 or transfected with an empty vector. Data represent the percentage of positive cells of paired analyzed samples of control or BMP4-transfected TKI-sensitive KCL22. (D) CD34+ cells from newly diagnosed CP patients treated or not in serum-free media with IM at 1 or 2 µM, and with or without sBMPR1b. After 5 days of culture, TWIST-1 gene expression was analyzed. BMPR1b expression was modulated in the KCL22 cell line by transfection with an empty vector as a control or a BMPR1b-expressing sequence to induce its expression in KCL22-sensitive cells (E) or using a vector containing short hairpine (sh) sequence against BMPR1b to decrease its level in KCL22-resistant cells (F). After puromycin selection, cells were incubated with 1% fetal calf serum in the presence of IM at the indicated dose. After 3 days of culture, cell proliferation and viability were determined by trypan blue staining. The percentage of proliferation was determined by reference to the untreated number of viable cells ± SEM from four experiments. (G) Model for BMPR1b-mediated TKI resistance of CML LSC. CT, control; NS, not significant (P > .05). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Discussion
Recently, technological breakthroughs have maximized the understanding of LSC heterogeneity. In CML, several SC subpopulations have been identified with distinct and dynamic proportions between diagnosis and TKI treatment and after treatment.1,2
In normal hematopoiesis, we have demonstrated that secreted BMPs regulate HSC cell fate,25,26 and we described an overproduction of BMP2 and BMP4 in the niche together with the overexpression of BMPR1b in primitive CML cells at diagnosis.17 Here, our data indicate that LSC BMPR1b+ subpopulations are specifically protected from TKI-induced apoptosis and persist in resistant CML patients. Current data, such as single-cell analysis,1,2,41 indicate that resistance could emerge from the heterogeneity of LSC subpopulations depending on the cues delivered by their microenvironment. It is then possible that residual BMPR1b-positive cells would persist under treatment despite responsive or nonresponsive status of the patient. After treatment resumption or because of secondary events occurring within this BMPR1b-expressing LSC subpopulation or within their BM microenvironment, BMPR1b-expressing LSC fraction can acquire a selective advantage to further proliferate and expand, leading to TKI escape. In particular, this could be promoted by a progressive increase in available BMP4 within the BM microenvironment or following autologous production set-up upon TKI treatment pressure. Indeed, activation of autocrine loops involving proteins/cytokines other than BMPs (IL-3, G-CSF) have been documented in untreated CML.42 Although at diagnosis we observed an excess of BMP2/BMP4 in the leukemic BM secreted by cell types other than LSC,17 we evidenced the existence of a BMP4-autocrine loop in the context of TKI resistance. There is mounting evidence that in addition to intrinsic events such as BCR-ABL expression, factors from the LSC niche play an important role in leukemia. This includes numerous signaling pathways, but varying evidence focuses on growth factors and cytokines such as IL-6, IL-1, CXCL12, macrophage inflammatory protein 1α, parathyroid hormone,43 vascular endothelial growth factor,44 and fibroblast growth factor 2.45-48 We showed that BMP2/4 initial deregulation evolves under TKI treatment toward a larger production of BMP4 from stromal cells and CD34+ leukemic cells themselves, indicating that long-term pressure of TKI treatment promotes evolving deregulation of cytokine pathways, as with BMPs. The direct involvement of BMP4 in TKI resistance through binding to BMPR1b+ LSC has been demonstrated using different approaches such as the use of sBMPR1b,17,40 which reversed the BMP2/4-mediated LSC amplification.
Computerized transcriptional correlation analyses on 140 CD34+-CP CML samples showed strong and significant correlation between BMP4 and BMPR1b and TWIST-1. TWIST-1 is overexpressed in CML primitive cells and implicated in the prediction of IM resistance in CP-CML patients.32 Long-term exposure to BMP2/4 upregulates TWIST-1 expression only in the presence of BCR-ABL. BMP4 or BMPR1b modulation in IM-sensitive or -resistant KCL22 cell lines38 confirmed the direct link between these different elements and identified TWIST-1 as a BMP pathway target. Our data, which established the implementation of a BMP-autocrine loop following long-term TKI treatment, may explain the specific BCR-ABL+ LSC survival with a megakaryocytic/erythroid commitment,1 because we identified BMP4 as a major regulator of megakaryocytic lineage25 and a cofactor of erythroid differentiation.26 Indeed, malignant BCR-ABL−-SCs and persisting subpopulations of BCR-ABL+-SCs with a megakaryocytic/erythroid program profile have been identified,1,2 both displaying modulation of the transforming growth factor β (TGFβ) pathway. This is also in line with data identifying the thrombopoietin (TPO) signaling pathway as one driver of LCS heterogeneity in CML.49 Indeed, we demonstrated, in a normal context, that TPO regulates megakaryopoiesis through the upregulation of BMP4 signaling.25 The role of the BMP pathway in maintenance of normal or transformed stem cell self-renewal is well documented in different systems. For example, the cross-talk between BMP and WNT pathways, which both converge to regulate the HOX gene expression through β-catenin, is involved in increasing CML-LSC self-renewal capacities.50 In addition, in a normal context, we showed cross-talk between BMP4 and MEK or JAK/STAT25 signaling, important pathways in CML TKI treatment survival.41,51 Lastly, deregulation of the BMP pathway that persists under TKI could contribute to the enrichment of the TGFβ signature in both BCR-ABL+ SCs and BCR-ABL− BM cells of resistant patients, as revealed by single-cell transcriptomic data.2
Concomitantly with arguments from the literature, our data indicate that the use of a continuous-treatment pressure may result in the development of a growth factor autocrine loop within the highly plastic LSC compartments. This situation could reflect evolution of CML over time and upon treatment adaptation of the leukemic niche, as has been suggested.52 This could contribute to the TKI survival of a subfraction of LSC, independent of BCR-ABL and dependent on growth factor autocrine loops, and their persistence in resistant patients (Figure 6G). In conclusion, despite TKI treatment, the BMP pathway remained deregulated in CP-CML at both intrinsic and extrinsic leukemic levels, especially in resistant cells. This fraction of LSC BM-resident surviving LSCs may depend on the installation of a BMP4/BMPR1b autocrine loop involving TWIST-1 overexpression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Morisset (Centre Hospitalier Lyon Sud, Lyon, France) for statistics, A. Beaumont and I. Durand (Cytometry Facility, Cancer Research Centre of Lyon, Lyon, France) for their technical assistance, B. White for English editing, M. Sobh for editorial assistance, and Servier Art for the illustrations.
This work was supported by grants from Novartis-Pharma France; patients’ associations Anim’ Montbernier (F.E.N., V.M-S.) and Leucémie Myéloïde Chronique (LMC) France (Mina Daban, president); Fondation de France (2014-0047501); Association Laurette Fugain (ALF2014-03); Ligue Contre le Cancer (Haute Savoie, Loire, Puy de Dôme, and Rhone); and Région Rhône-Alpes Auvergne (C-MIRA14.007020). Fellowships were obtained from the French government (B.L.) and from Ligue Contre le Cancer (E.G., B.L).
Authorship
Contribution: E.G., B.L., S.J., S.G., B.G., and T.V. performed experiments and data analysis; F.E.N. followed up with patients, analyzed data, provided patients and donors samples, and wrote the manuscript; V.M.-S. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Véronique Maguer-Satta, Cancer Research Center of Lyon-CRCL, U1052-UMR5286, 28 rue Laennec, 69373 Lyon Cedex 08, France; e-mail: veronique.maguer-satta@lyon.unicancer.fr; or Franck E. Nicolini, Hematology Department, Centre Léon Bérard, 28 rue Laënnec, 69373 Lyon Cédex 08, France; e-mail: franck-emmanuel.nicolini@lyon.unicancer.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal