Abstract
Traditionally, in vitro flow chamber experiments and in vivo arterial thrombosis studies have been proved to be of vital importance to elucidate the mechanisms of platelet thrombus formation after vessel wall injury. In recent years, it has become clear that platelets also act as modulators of inflammatory processes, such as atherosclerosis. A key element herein is the complex cross talk between platelets, the coagulation system, leukocytes, and the activated endothelium. This review provides insight into the platelet-endothelial interface, based on in vitro flow chamber studies and cross referenced with in vivo thrombosis studies. The main mechanisms of platelet interaction with the activated endothelium encompass (1) platelet rolling via interaction of platelet glycoprotein Ib-IX-V with endothelial-released von Willebrand factor with a supporting role for the P-selectin/P-selectin glycoprotein ligand 1 axis, followed by (2) firm platelet adhesion to the endothelium via interaction of platelet αIIbβ3 with endothelial αvβ3 and intercellular adhesion molecule 1, and (3) a stimulatory role for thrombin, the thrombospondin-1/CD36 axis and cyclooxygenase 1 in subsequent platelet activation and stable thrombus formation. In addition, the molecular mechanisms underlying the stimulatory effect of platelets on leukocyte transendothelial migration, a key mediator of atheroprogression, are discussed. Throughout the review, emphasis is placed on recommendations for setting up, reporting, interpreting, and comparing endothelial-lined flow chamber studies and suggestions for future studies.
Introduction
Under physiological conditions, platelets, the coagulation system and the endothelium function in favor of each other. Platelets maintain the integrity of the endothelium, while this endothelium releases nitric oxide and prostacyclin and expresses the ectonucleotidase CD39 to keep platelets in a resting state.1 Additionally, platelets arrest bleeding (hemostasis) after vessel wall injury by forming a thrombus. Our understanding of the reactions leading to thrombus formation after injury of a healthy, nonatherosclerotic artery has been largely based on in vitro flow chamber studies with human and mouse blood and in vivo mouse studies, with the main processes being platelet activation, thrombin generation, and fibrin clot formation.2 In spite of different characteristics of mouse platelets in comparison with human platelets, such as the lack of the protease activated receptor-1 (PAR-1) receptor, an approximately twofold higher plasma concentration, and a shorter life span,3 the thrombus forming process of human and mouse platelets over surfaces coated with collagen occurs via similar mechanisms.4,5 Moreover, the experimental outcome of these flow chamber experiments correlates well with the outcome of in vivo arterial thrombosis models.6 Commonly used schemes of hemostasis start with platelet adhesion to von Willebrand factor (VWF), which is facilitated by the high shear rate at the arterial vessel wall. Subsequent activation of platelets is promoted by the release of paracrine mediators, like adenosine diphosphate (ADP) and thromboxane.2 Thrombin, generated at the surface of a subpopulation of procoagulant platelets and at disrupted subendothelial membranes, activates platelets and cleaves fibrinogen into fibrin,5,7 promoting thrombus growth and stabilization.4 Thrombin also activates a negative feedback loop to suppress its own generation by binding to endothelial-expressed thrombomodulin. Thrombomodulin-bound thrombin proteolytically activates the anticoagulant factor protein C, which is also bound to the endothelium via endothelial protein C receptor. Activated protein C inactivates coagulation factors Va and VIIIa leading to dampening of thrombin generation.2
Under inflammatory conditions, the cross talk between platelets, the coagulation system, and the endothelium is no longer favorable but is thought to exacerbate inflammation.8,9 Although some players in this cross talk have been identified, the puzzle is far from complete. A key aspect in the cross talk appears to be the stimulatory effect of platelets and platelet-derived microvesicles on leukocyte transendothelial migration, which is a known mediator of atheroprogression. Platelets interact via P-selectin with leukocyte P-selectin glycoprotein ligand 1 (PSGL-1). The platelet-leukocyte interaction is strengthened via interaction of leukocyte αMβ2 integrin with platelet receptors glycoprotein Ib (GPIb), junctional adhesion molecule A or junctional adhesion molecule C, or αIIbβ3 (via fibrinogen).10-13 Vice versa, leukocytes can secrete substances, like human neutrophil peptides,14 that induce platelet activation. An important consequence of these cellular interactions is platelet-, leukocyte-, and endothelial cell (EC)–granular secretion, leading to the release of soluble constituents and to an increased surface expression of membrane receptors, which fuels a complex and vicious proinflammatory circle. Interestingly, platelets can also actively deposit granular constituents on the inflamed endothelium, as demonstrated for the chemokines CCL5 (RANTES) and CXCL4 (platelet factor 4). The presence of CXCL4 was found to enhance the CCL5-induced arrest of leukocytes on activated ECs.15 For a more in-depth description about the role of chemokines in inflammatory and thrombotic processes, the authors refer to recent reviews by van der Vorst et al,16 Koenen,17 and Soehlein et al.18
A true challenge for the future is not to obtain new pieces of the puzzle, but to integrate the mechanistic evidence and identify the main players in this cross talk in the human system. This review article provides a means to do so with regard to the direct cell-cell interactions between platelets and the endothelium.
Status quo of endothelial-lined flow chamber studies: blood compartment
Flow chamber studies into mechanisms of platelet-endothelial interactions have been rapidly emerging over recent years. Although technical advances in microfluidics have increased flexibility in flow chamber design, this has also hampered standardization and comparison of results between studies.19,20 In this paragraph, key variables between flow chamber studies are listed, and it is explained how these variables could influence the platelet-endothelial interface (Figure 1). Strikingly, a major potential source of variation, flow channel dimension, is only reported in a minority of papers. Grosso modo platelets can undergo 2 types of interactions: platelet-platelet interactions and platelet-surface interactions. The transition between situations in which platelet-surface interactions or platelet-platelet interactions dominate is a function of channel size and aspect ratio (height/width) with values of <0.2 are being recommended to achieve constant shear stress, and thus laminar flow, across the middle of the adhesive surface.20 Under these physiological conditions, red blood cells (RBCs) become centered in the middle of the vessel/flow chamber and push platelets towards the wall of the flow chamber (ie, so-called platelet margination). Importantly, RBCs may also influence the efficacy of antithrombotic agents. For example, the antithrombotic effects of the phosphodiesterase type 5 (PDE5) inhibitor dipyridamole are largely attributed to its inhibition of adenosine reuptake by RBCs and to a lesser extent to its inhibition of platelet PDE5.21 Hence, having RBCs present in the blood sample is of key importance to mimic the physiological process of platelet adhesion under flow, and, in general, superior to the use of platelet-rich plasma. The fact that there are no clear differences observed in study outcomes between studies with whole blood vs platelet-rich plasma (Table 1) does not impact the importance of having RBCs present, if only because several other factors also vary between these studies.
Key methodological variables in endothelial-lined flow chamber studies. Flexibility in flow chamber design has resulted in a range of different endothelial-lined flow chamber setups. Listed here are key variables between those studies: (1) Flow chamber dimensions: to achieve laminar flow, a height/width ratio of <0.2 is recommended. (2) Presence/absence of RBCs: RBCs are required for platelet margination and may influence the efficacy of certain antithrombotic agents, like dipyridamole. (3) Culturing of ECs under static or shear stress conditions: ECs change phenotype upon sensing mechanical stress; hence, culturing under shear stress is advised. (4) Presence/absence of Ca2+ and Mg2+: physiological Ca2+ and Mg2+ levels are required for integrin function and for coagulation to take place. (5) Different EC sources: distinctive endothelial heterogeneity throughout the vasculature illustrates the importance of using an EC source appropriate to the research question. In addition, the expression level of several proteins that are involved in thrombus formation is affected by the passage number. (6) Confluency of EC layer: should be reported as a lack of confluency may trigger thrombus formation, because of exposure of subendothelial matrix components to the blood. Additional clarification is provided in the text. To enable comparison between studies, we encourage reporting the variables specified in this figure.
Key methodological variables in endothelial-lined flow chamber studies. Flexibility in flow chamber design has resulted in a range of different endothelial-lined flow chamber setups. Listed here are key variables between those studies: (1) Flow chamber dimensions: to achieve laminar flow, a height/width ratio of <0.2 is recommended. (2) Presence/absence of RBCs: RBCs are required for platelet margination and may influence the efficacy of certain antithrombotic agents, like dipyridamole. (3) Culturing of ECs under static or shear stress conditions: ECs change phenotype upon sensing mechanical stress; hence, culturing under shear stress is advised. (4) Presence/absence of Ca2+ and Mg2+: physiological Ca2+ and Mg2+ levels are required for integrin function and for coagulation to take place. (5) Different EC sources: distinctive endothelial heterogeneity throughout the vasculature illustrates the importance of using an EC source appropriate to the research question. In addition, the expression level of several proteins that are involved in thrombus formation is affected by the passage number. (6) Confluency of EC layer: should be reported as a lack of confluency may trigger thrombus formation, because of exposure of subendothelial matrix components to the blood. Additional clarification is provided in the text. To enable comparison between studies, we encourage reporting the variables specified in this figure.
Reported involvement of platelet receptors and downstream signaling mediators in thrombus formation on inflamed or damaged endothelium
| Target . | Intervention . | Flow chamber . | Shear rate . | EC source . | EC treatment . | Platelet source . | Effect intervention on thrombus formation . | Reference . |
|---|---|---|---|---|---|---|---|---|
| α5 | CBL497 Ab | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings = | 44* |
| α6 | GoH3 Ab | Parallel plate | 150/s | HUVECs | Heat | WB | Platelet aggregate volume ↓ | 40 |
| αvβ3 | LM609 Ab | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings = , VWF strings = | 44* |
| αIIbβ3/αvβ3 | Abciximab (7E3, ReoPro) | Parallel plate | 1000/s | HUVECs | IFN-γ + TNF-α | WPs | Platelet SAC ↓ | 59 |
| Abciximab (7E3, ReoPro) | Parallel plate | 50 dynes/cm2 | pPAECs | Serum | WB | Platelet SAC ↓ | 46 | |
| Abciximab (7E3, ReoPro) | Parallel plate | 750/s | HUVECs, fixated | TNF-α | WB | Platelet SAC ↓ | 34 | |
| Abciximab (7E3, ReoPro) | Capillary, rectangular | 100/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | Platelet SAC ↓ | 58 | |
| Abciximab (7E3, ReoPro) | Bioflux 1000 | 5 dynes/cm2 | PAECs | Xenoactivation | WB | Platelet SAC, platelet aggregate volume ↓ | 25 | |
| Abciximab (7E3, ReoPro) | Parallel plate | 150/s | HUVECs | Heat | WB | Platelet aggregate volume ↓ | 40 | |
| β3 function neutralizing Ab | Capillary, rectangular | 140/s | pHUVECs + pSMCs | TGF-β1 | WB | Platelet SAC ↓ | 47 | |
| αIIbβ3 | Eptifibatide (Integrilin) | μ-slide VI | 50/s | LECs | None | WB | Stable platelet-EC interactions = , platelet SAC ↑ | 71 |
| Eptifibatide (Integrilin) | Branching microchannel | 1-4, 10-40 dynes/cm2 | HUVECs | STX-2 | WB | Platelet aggregate volume ↓, channel occlusion ↓ | 60 | |
| Eptifibatide (Integrilin) | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB | Channel occlusion ↓ | 26 | |
| Lotrafiban | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC = | 71 | |
| MA-16N7C2 Ab | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation = , number of adhered platelets ↓ | 43 | |
| P2 mAb | SPAA | 170/s | HUVECs | None | PRP, ADP stimulated | Platelet SAC ↓ | 57 | |
| P2 mAb | Cone and plate, adapted | 250/s | pBAECs | None | PRP, TRAP-6 stimulated | Number of adhered platelets ↓ | 56 | |
| RGDS peptide | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings ↓, VWF strings ↓ | 44* | |
| RGDS peptide | SPAA | 170/s | HUVECs | None | PRP, ADP stimulated | Platelet SAC ↓ | 57 | |
| Ro 43-8857 | SPAA | 170/s | HUVECs | None | PRP, ADP stimulated | Platelet SAC ↓ | 57 | |
| TAK-029 | Not indicated | 10/s | HBECs | None | PRP, ADP stimulated | Number of adhered platelets ↓ | 85 | |
| TAK-029 | Not indicated | 10/s | HAECs | None | PRP, ADP stimulated | Number of adhered platelets ↓ | 86 | |
| ADAMTS13 | ADAMTS13 function neutralizing pAb | Capillary, rectangular | 400/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | VWF strings ↑, platelets in VWF strings ↑ | 58 |
| C5 | Eculizumab | Bioflux 200 | 3, 10 dynes/cm2 | pPAECs | Xenoactivation | PRP | Platelet SAC ↓ | 23 |
| Eculizumab | Bioflux 200 | 3, 10 dynes/cm2 | GTKO/hCD46-pPAECs | Xenoactivation | PRP | Platelet SAC ↓ | 23 | |
| CD36 | FA6.152 mAb | Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB, rec. | Platelet SAC ↓ | 65 |
| CD47 | Agonist: 4N1k | Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB | Platelet SAC ↑ | 65 |
| B6H12 mAb | Parallel plate, Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB | Platelet SAC ↓ | 65 | |
| Cd47−/− | Parallel plate | 100/s | Ea.hy926 | TNF-α | WB, mouse | Platelet SAC ↓ | 65 | |
| CLEC-2 | Clec2−/− | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC ↓ | 71 |
| COX-1 | Aspirin | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB | Channel occlusion ↓ | 26 |
| F-actin | Cytochalasin D | μ-slide VI | 50/s | LECs | None | WB | Platelet SAC ↓ | 71 |
| GPIbα | 6D1 Ab | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings ↓, VWF strings = | 44* |
| AK2, SZ2 mAb’s | Parallel plate | 2 dynes/cm2 | pHUVECs | Histamine | WPs + RBCs | Platelet translocation ↓ | 33 | |
| AK2 mAb | Parallel plate | 2.5 dynes/cm2 | pHUVECs | Histamine | WPs | Platelet-VWF strings ↓ | 39 | |
| AK2 mAb | Branching microchannel | 10-30 dynes/cm2 | HUVECs | Shear | WB, rec. | Platelet adhesion ↓ | 45 | |
| ATA | Parallel plate | 50 dynes/cm2 | pPAECs | Serum | WB | Platelet SAC ↓ | 46 | |
| G19H10 Ab | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| GPIbα function blocking Ab | Parallel plate | 150/s | HUVECs | Heat | WB | Platelet aggregate volume ↓ | 40 | |
| GPIbα function neutralizing Ab | Capillary, rectangular | 140/s | pHUVECs + pSMCs | TGF-β1 | WB | Platelet SAC ↓ | 47 | |
| GUR20-5 Ab | Not indicated | 10/s | HBECs | None | PRP, ADP stimulated | Number of adhered platelets = | 85 | |
| GUR20-5 Ab | Not indicated | 10/s | HAECs | None | PRP, ADP stimulated | Number of adhered platelets = | 86 | |
| IL4-R/Iba | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB, mouse | Channel occlusion ↓ | 26 | |
| SZ2 mAb | Parallel plate | 1000/s | HUVECs | IFN-γ + TNF-α | WPs | Platelet SAC ↓ | 59 | |
| SZ2 mAb | Capillary, rectangular | 100/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | Platelet SAC ↓ | 58 | |
| PAR-1 | Agonist: TRAP-6 | Parallel plate | 310/s | Ea.hy926 | None | WB, rec. | Number of adhered platelets ↑ | 48 |
| Agonist: TRAP-6 | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↑ | 43 | |
| P2X1 | Agonist: ATP | Rectangular | Not indicated | BPAECs | None | WPs | Number of adhered platelets ↓ (ATP <50 nM), ↑ (ATP >100 nM) | 64 |
| Agonist: RBC derived ATP | Rectangular | Not indicated | BPAECs | None | WPs + RBCs | Number of adhered platelets ↓ | 64 | |
| Glibenclamide | Rectangular | Not indicated | BPAECs | None | WPs + RBCs | Number of adhered platelets ↑ | 64 | |
| PDE/AR | Theophylline | Capillary, rectangular | 140/s | pHUVECs | TGF-β1 | WB | Platelet SAC ↓ | 47 |
| P-selectin | Agonist: soluble P-selectin | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings ↓, VWF strings = | 44* |
| AK-6 mAb | Cone and plate, adapted | 250/s | pBAECs | None | PRP, TRAP-6 stimulated | Number of adhered platelets ↓ | 56 | |
| CLB-Thromb/6 mAb | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| pAb | Parallel plate | 2 dynes/cm2 | pHUVECs | Histamine | WPs + RBCs | Platelet translocation ↓ | 33 | |
| pAb | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings = , VWF strings = | 44* | |
| WASP12.2 | Not indicated | 6 dynes/cm2 | HUVECs | TNF-α | WPs | Number of adherent platelets ↓ | 50 | |
| Rac | EHT1864 | μ-slide VI | 50/s | LECs | None | WB | Platelet SAC = | 71 |
| SIRPα | SE5A5 mAb | Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB, rec. | Platelet SAC = | 65 |
| Src | Dasatinib | μ-slide VI | 50/s | LECs | None | WB | Stable platelet-EC interactions ↓, platelet SAC ↓ | 71 |
| Dasatinib | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC ↓ | 71 | |
| Syk | PRT060318 | μ-slide VI | 50/s | LECs | None | WB | Stable platelet-EC interactions ↓, platelet SAC = | 71 |
| Syk−/− | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC ↓ | 71 | |
| Thrombin | Bivalirudin | Bioflux 1000 | 5 dynes/cm2 | PAECs | Xenoactivation | WB | Platelet SAC ↓, platelet aggregate volume ↓ | 25 |
| Hirudin, heparin | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB | Channel occlusion ↓ | 26 | |
| Ro 46-6240, hirudin, heparin | Parallel plate | 65/s | pHUVECs | TNF-α | WB | Number of platelet aggregates ↓ | 24 | |
| VCAM-1 | 1.4C3 Ab | Capillary, rectangular | 100/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | Platelet SAC = | 58 |
| VWF | 6G1 mAb | Parallel plate | 2.5 dynes/cm2 | pHUVECs | Histamine | WPs | Platelet-VWF strings ↓ | 39 |
| AJvW-2 Ab | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| AJvW-2 Ab | Not indicated | 24 dynes/cm2 | Aorta, rabbit | Cholesterol diet | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| VWD type 3 | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB, rec. | Channel occlusion ↓ | 26 |
| Target . | Intervention . | Flow chamber . | Shear rate . | EC source . | EC treatment . | Platelet source . | Effect intervention on thrombus formation . | Reference . |
|---|---|---|---|---|---|---|---|---|
| α5 | CBL497 Ab | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings = | 44* |
| α6 | GoH3 Ab | Parallel plate | 150/s | HUVECs | Heat | WB | Platelet aggregate volume ↓ | 40 |
| αvβ3 | LM609 Ab | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings = , VWF strings = | 44* |
| αIIbβ3/αvβ3 | Abciximab (7E3, ReoPro) | Parallel plate | 1000/s | HUVECs | IFN-γ + TNF-α | WPs | Platelet SAC ↓ | 59 |
| Abciximab (7E3, ReoPro) | Parallel plate | 50 dynes/cm2 | pPAECs | Serum | WB | Platelet SAC ↓ | 46 | |
| Abciximab (7E3, ReoPro) | Parallel plate | 750/s | HUVECs, fixated | TNF-α | WB | Platelet SAC ↓ | 34 | |
| Abciximab (7E3, ReoPro) | Capillary, rectangular | 100/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | Platelet SAC ↓ | 58 | |
| Abciximab (7E3, ReoPro) | Bioflux 1000 | 5 dynes/cm2 | PAECs | Xenoactivation | WB | Platelet SAC, platelet aggregate volume ↓ | 25 | |
| Abciximab (7E3, ReoPro) | Parallel plate | 150/s | HUVECs | Heat | WB | Platelet aggregate volume ↓ | 40 | |
| β3 function neutralizing Ab | Capillary, rectangular | 140/s | pHUVECs + pSMCs | TGF-β1 | WB | Platelet SAC ↓ | 47 | |
| αIIbβ3 | Eptifibatide (Integrilin) | μ-slide VI | 50/s | LECs | None | WB | Stable platelet-EC interactions = , platelet SAC ↑ | 71 |
| Eptifibatide (Integrilin) | Branching microchannel | 1-4, 10-40 dynes/cm2 | HUVECs | STX-2 | WB | Platelet aggregate volume ↓, channel occlusion ↓ | 60 | |
| Eptifibatide (Integrilin) | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB | Channel occlusion ↓ | 26 | |
| Lotrafiban | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC = | 71 | |
| MA-16N7C2 Ab | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation = , number of adhered platelets ↓ | 43 | |
| P2 mAb | SPAA | 170/s | HUVECs | None | PRP, ADP stimulated | Platelet SAC ↓ | 57 | |
| P2 mAb | Cone and plate, adapted | 250/s | pBAECs | None | PRP, TRAP-6 stimulated | Number of adhered platelets ↓ | 56 | |
| RGDS peptide | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings ↓, VWF strings ↓ | 44* | |
| RGDS peptide | SPAA | 170/s | HUVECs | None | PRP, ADP stimulated | Platelet SAC ↓ | 57 | |
| Ro 43-8857 | SPAA | 170/s | HUVECs | None | PRP, ADP stimulated | Platelet SAC ↓ | 57 | |
| TAK-029 | Not indicated | 10/s | HBECs | None | PRP, ADP stimulated | Number of adhered platelets ↓ | 85 | |
| TAK-029 | Not indicated | 10/s | HAECs | None | PRP, ADP stimulated | Number of adhered platelets ↓ | 86 | |
| ADAMTS13 | ADAMTS13 function neutralizing pAb | Capillary, rectangular | 400/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | VWF strings ↑, platelets in VWF strings ↑ | 58 |
| C5 | Eculizumab | Bioflux 200 | 3, 10 dynes/cm2 | pPAECs | Xenoactivation | PRP | Platelet SAC ↓ | 23 |
| Eculizumab | Bioflux 200 | 3, 10 dynes/cm2 | GTKO/hCD46-pPAECs | Xenoactivation | PRP | Platelet SAC ↓ | 23 | |
| CD36 | FA6.152 mAb | Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB, rec. | Platelet SAC ↓ | 65 |
| CD47 | Agonist: 4N1k | Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB | Platelet SAC ↑ | 65 |
| B6H12 mAb | Parallel plate, Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB | Platelet SAC ↓ | 65 | |
| Cd47−/− | Parallel plate | 100/s | Ea.hy926 | TNF-α | WB, mouse | Platelet SAC ↓ | 65 | |
| CLEC-2 | Clec2−/− | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC ↓ | 71 |
| COX-1 | Aspirin | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB | Channel occlusion ↓ | 26 |
| F-actin | Cytochalasin D | μ-slide VI | 50/s | LECs | None | WB | Platelet SAC ↓ | 71 |
| GPIbα | 6D1 Ab | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings ↓, VWF strings = | 44* |
| AK2, SZ2 mAb’s | Parallel plate | 2 dynes/cm2 | pHUVECs | Histamine | WPs + RBCs | Platelet translocation ↓ | 33 | |
| AK2 mAb | Parallel plate | 2.5 dynes/cm2 | pHUVECs | Histamine | WPs | Platelet-VWF strings ↓ | 39 | |
| AK2 mAb | Branching microchannel | 10-30 dynes/cm2 | HUVECs | Shear | WB, rec. | Platelet adhesion ↓ | 45 | |
| ATA | Parallel plate | 50 dynes/cm2 | pPAECs | Serum | WB | Platelet SAC ↓ | 46 | |
| G19H10 Ab | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| GPIbα function blocking Ab | Parallel plate | 150/s | HUVECs | Heat | WB | Platelet aggregate volume ↓ | 40 | |
| GPIbα function neutralizing Ab | Capillary, rectangular | 140/s | pHUVECs + pSMCs | TGF-β1 | WB | Platelet SAC ↓ | 47 | |
| GUR20-5 Ab | Not indicated | 10/s | HBECs | None | PRP, ADP stimulated | Number of adhered platelets = | 85 | |
| GUR20-5 Ab | Not indicated | 10/s | HAECs | None | PRP, ADP stimulated | Number of adhered platelets = | 86 | |
| IL4-R/Iba | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB, mouse | Channel occlusion ↓ | 26 | |
| SZ2 mAb | Parallel plate | 1000/s | HUVECs | IFN-γ + TNF-α | WPs | Platelet SAC ↓ | 59 | |
| SZ2 mAb | Capillary, rectangular | 100/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | Platelet SAC ↓ | 58 | |
| PAR-1 | Agonist: TRAP-6 | Parallel plate | 310/s | Ea.hy926 | None | WB, rec. | Number of adhered platelets ↑ | 48 |
| Agonist: TRAP-6 | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↑ | 43 | |
| P2X1 | Agonist: ATP | Rectangular | Not indicated | BPAECs | None | WPs | Number of adhered platelets ↓ (ATP <50 nM), ↑ (ATP >100 nM) | 64 |
| Agonist: RBC derived ATP | Rectangular | Not indicated | BPAECs | None | WPs + RBCs | Number of adhered platelets ↓ | 64 | |
| Glibenclamide | Rectangular | Not indicated | BPAECs | None | WPs + RBCs | Number of adhered platelets ↑ | 64 | |
| PDE/AR | Theophylline | Capillary, rectangular | 140/s | pHUVECs | TGF-β1 | WB | Platelet SAC ↓ | 47 |
| P-selectin | Agonist: soluble P-selectin | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings ↓, VWF strings = | 44* |
| AK-6 mAb | Cone and plate, adapted | 250/s | pBAECs | None | PRP, TRAP-6 stimulated | Number of adhered platelets ↓ | 56 | |
| CLB-Thromb/6 mAb | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| pAb | Parallel plate | 2 dynes/cm2 | pHUVECs | Histamine | WPs + RBCs | Platelet translocation ↓ | 33 | |
| pAb | Parallel plate | 2.5 dynes/cm2 | HUVECs | Histamine | WPs, fixed | Platelet-VWF strings = , VWF strings = | 44* | |
| WASP12.2 | Not indicated | 6 dynes/cm2 | HUVECs | TNF-α | WPs | Number of adherent platelets ↓ | 50 | |
| Rac | EHT1864 | μ-slide VI | 50/s | LECs | None | WB | Platelet SAC = | 71 |
| SIRPα | SE5A5 mAb | Laboratory-Tek 1 | 100/s | Ea.hy926 | TNF-α | WB, rec. | Platelet SAC = | 65 |
| Src | Dasatinib | μ-slide VI | 50/s | LECs | None | WB | Stable platelet-EC interactions ↓, platelet SAC ↓ | 71 |
| Dasatinib | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC ↓ | 71 | |
| Syk | PRT060318 | μ-slide VI | 50/s | LECs | None | WB | Stable platelet-EC interactions ↓, platelet SAC = | 71 |
| Syk−/− | μ-slide VI | 50/s | LECs | None | WB, mouse | Platelet SAC ↓ | 71 | |
| Thrombin | Bivalirudin | Bioflux 1000 | 5 dynes/cm2 | PAECs | Xenoactivation | WB | Platelet SAC ↓, platelet aggregate volume ↓ | 25 |
| Hirudin, heparin | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB | Channel occlusion ↓ | 26 | |
| Ro 46-6240, hirudin, heparin | Parallel plate | 65/s | pHUVECs | TNF-α | WB | Number of platelet aggregates ↓ | 24 | |
| VCAM-1 | 1.4C3 Ab | Capillary, rectangular | 100/s | pHUVECs | TNF-α + TGF-β | WPs + RBCs | Platelet SAC = | 58 |
| VWF | 6G1 mAb | Parallel plate | 2.5 dynes/cm2 | pHUVECs | Histamine | WPs | Platelet-VWF strings ↓ | 39 |
| AJvW-2 Ab | Parallel plate | 24 dynes/cm2 | Ea.hy926 | PLPC | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| AJvW-2 Ab | Not indicated | 24 dynes/cm2 | Aorta, rabbit | Cholesterol diet | WB, rec.† | Platelet translocation ↓, number of adhered platelets ↓ | 43 | |
| VWD type 3 | Branching microchannel | 4 dynes/cm2 | HUVECs | FeCl3 | WB, rec. | Channel occlusion ↓ | 26 |
All platelet sources are of human origin unless indicated otherwise. Decreased (↓); no change (=); increased (↑).
Ab, antibody; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; AR, adenosine receptor; ATA, aurin tricarboxylic acid; ATP, adenosine triphosphate; BPAEC, bovine pulmonary artery endothelial cell; C5, complement component; CLEC-2, c-type lectinlike receptor 2; COX-1, cyclooxygenase 1; GTKO/hCD46-pPAEC, alpha1.3-galactosyltransferase deficient/human CD46 transgenic-primary porcine aortic endothelial cell; HAEC, human aortic endothelial cell; HBEC, human brain microvascular endothelial cell; HUVEC, human umbilical vein endothelial cell; IL, interleukin; INF-γ, interferon-γ; LEC, lymphatic endothelial cell; mAb, monoclonal antibody; pAb, polyclonal antibody; PAEC, porcine aortic endothelial cell; pBAEC, primary bovine aortic endothelial cell; pHUVEC, primary human umbilical vein endothelial cell; PLPC, palmitoyllysophosphatidylcholine; PLCγ2, phosholipase γ2; PMA, phorbol-12-myristate-13-acetate; pPAEC, primary porcine aortic endothelial cell; PRP, platelet-rich plasma; pSMC, primary smooth muscle cell; rec., reconstituted; RGDS, arginine glycine asparagine serine; SAC, surface area coverage; SIRPα, signal regulatory protein α; SPAA, stagnation point flow adhesio-aggregometer; STX-2, shiga toxin 2; TGF-β1, transforming growth factor β1; TNF-α, tumor necrosis factor α; TRAP-6, thrombin receptor-activating peptide 6; VCAM-1, vascular cell adhesion molecule 1; VWD, von Willebrand disease; WB, whole blood; WP, washed platelet.
No statistics applied.
Spiked with TRAP-6-activated platelets.
The type of anticoagulant used and in particular whether the anticoagulant does (eg, citrate) or does not (eg, hirudin, PPACK) remove divalent cations forms another potential source of variation. The adhesive properties of integrins α2β1 and αIIbβ3 are quite sensitive to the extracellular levels of divalent cations, because of their Ca2+ and/or Mg2+ binding domains, which control ligand binding.22 Indeed, Rataj et al23 reported reduced thrombus coverage in the presence of citrate when compared with heparin anticoagulated whole blood. Even with physiological Ca2+ and Mg2+ levels present (eg, in heparin-anticoagulated blood), one has to be aware that, although integrin function is no longer impaired, the coagulation process is still blocked. As a consequence, the role of thrombin, a key protease in the coagulation cascade, and also a potent platelet activator, is not taken into account. Importantly, thrombin, and coagulation factors Xa, activated protein C, and the tissue factor/factor VIIa complex, also interact with PAR-1 on the endothelium, thereby promoting the release of endothelial-derived VWF and the exposure of tissue factor and P-selectin at the endothelial surface.2 Using a method where native non-anticoagulated whole blood was perfused directly from the vein of the donor through the flow chamber, Kirchhofer et al24 were the first to demonstrate that inhibition of thrombin led to greatly reduced platelet deposition, and fibrin deposition, on TNF-α–stimulated ECs under flow. Since then, to the best of our knowledge, only 2 groups have studied the contribution of the coagulation system to thrombus formation and/or channel occlusion in endothelium-lined flow chambers. Harris et al25 found a strong reduction in platelet surface area coverage and thrombus volume upon treatment of heparinized whole blood with the thrombin inhibitor bivalirudin, suggesting that anticoagulation with heparin did not suffice to block thrombin generation in this model where human blood was perfused over porcine ECs. Ciciliano et al26 recalcified citrated blood to allow coagulation to occur and observed reduced channel occlusion with hirudin and heparin in this setup. In contrast, in collagen-lined flow chambers the interplay between coagulation and platelets has been more extensively characterized, as reviewed elsewhere.5 The variables reported here (Figure 1) are not an exhaustive list, and other differences affect results, as reported elsewhere.27-29
Status quo of endothelial-lined flow chamber studies: endothelial compartment
Regarding the endothelial compartment, the following key aspects often vary between studies: culturing of ECs under shear vs static conditions, the type of stimulus used to trigger EC activation, the source and passage number of ECs, and the confluency of the EC layer (Figure 1). ECs sense mechanical stresses through several proteins that are also involved in the interaction of ECs with platelets and leukocytes, for example, β2 and β3 integrins, platelet and endothelial cell adhesion molecule 1, vascular endothelial cadherin, G-protein coupled receptors, and the cytoskeleton itself,30 triggering a differential regulation of ∼3% to 6% of endothelial genes.31 Following a shear stress transcriptional response, initiated by these receptors, 3 major types of responses can occur in ECs: (1) a detrimental, immediate response leading to the induction of endothelial activation; (2) a protective, chronic response causing suppression of endothelial activation, anti-inflammatory effectors, and morphologic adaptation to flow; or (3) a dysfunctional response to disturbed stresses leading to endothelial activation, inflammation, and atherogenesis.32 TNF-α is the most frequently used EC stimulus in flow chamber studies (supplemental Table 1, available on the Blood Web site). In response, ECs release VWF and present several effector proteins on their membrane, such as E-selectin, P-selectin, intercellular adhesion molecule 1 (ICAM-1), VCAM-1, and tissue factor.33,34 Alternatively, PMA, TGF-β1, STX-2, IFN-γ, cholesterol, FeCl3, and heat have been used to activate or damage the endothelium (supplemental Table 1). To our knowledge, no comprehensive comparison of cellular changes in ECs has been made between these agonists.
Of those genes that are influenced by the shear stress transcriptional response, the following nonexhaustive subset is involved in hemostasis and thrombosis: thrombomodulin, tissue factor, endothelial nitric oxide synthase, platelet and endothelial cell adhesion molecule 1, VCAM-1, and P-selectin.31 By and large, a pattern of protective antithrombotic changes can be seen when the ECs are cultured under physiological laminar flow patterns, while changes in flow profiles during the experiment are prothrombotic and proatherogenic.31 Without flow, the long-term shear induced alterations to EC protein expression and phenotype will likely not present.30 Altogether, the previously mentioned points advocate for culturing ECs under flow conditions that are representative for the vessel and the underlying (patho)physiology. Of note, not only shear stress, but also the interaction of platelets35 or platelet-derived microRNAs36,37 with ECs can modulate EC gene expression.
It is important to note that there is distinctive endothelial heterogeneity throughout the vasculature, including differences in tissue factor pathway inhibitor (predominantly in the capillaries), endothelial protein C receptor (large veins and arteries), endothelial nitric oxide synthase (found mostly arterial), and VWF (predominantly present in the veins under physiological conditions).38 The study by Dong et al39 represents one of few studies where different sources of ECs were examined in flow studies in a quantitative manner. They showed that the number of ultralong VWF strings is EC source dependent, with fewer ultralarge VWF strings forming on human coronary artery endothelial cells and human lung microvascular endothelial cells, both used after 4 to 5 passages, than on primary HUVECs and human umbilical artery endothelial cells. It remains to be determined whether the difference in passage number underlies the decreased ultralarge VWF strings formation in human coronary artery endothelial cells and human lung microvascular endothelial cells, or whether this is related to an inherent difference in VWF production.
Measurements of cell viability and confluence of the EC layer are another set of meaningful quality controls. Because platelets can interact with the extracellular matrix on which ECs are seeded, a lack of confluence can affect the outcome of the study. An example of the latter is provided by Sylman et al,40 who used heat to activate ECs and found that the large contribution of α6 to platelet aggregate formation in their model was because of exposure of the laminin-rich matrix after receding of the EC upon heat treatment. In addition, it is of relevance to report the passage number of the ECs used in the flow studies, because the expression level of several proteins that are involved in thrombus formation, such as galectin 1,41,42 are affected by the number of passages. Altogether, a main aim of this review is to increase awareness of the variables that may cause variation in flow chamber studies and to facilitate comparison between studies by reporting these variables.
Mechanisms of platelet rolling over activated endothelium
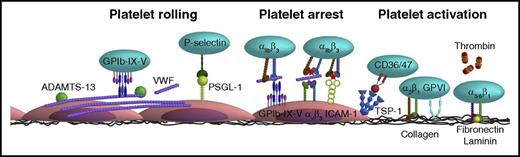
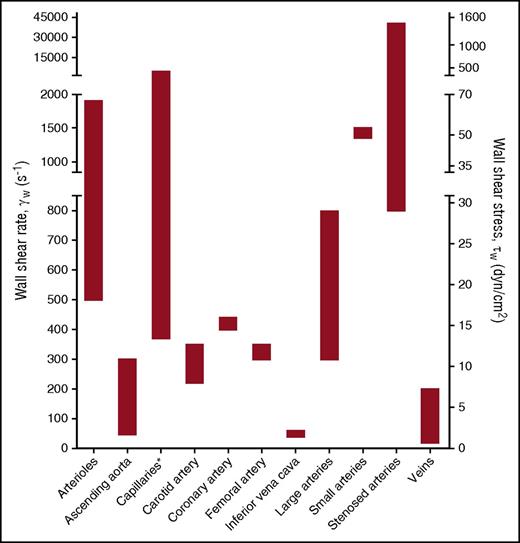
To integrate the experimental data obtained from flow chamber studies into a unifying model of platelet-EC interaction, in this review, experimental data from flow studies were combined with knowledge obtained from in vivo thrombosis studies. It is thought that the main mechanism of platelet rolling on the activated endothelium is mediated via interaction of platelet GPIb-IX-V complex with VWF,35 with a supporting role for the P-selectin/PSGL-1 axis (Figure 2). Inhibition of GPIbα, in flow chamber experiments, resulted in a shear-dependent reduction in platelet translocation and binding to VWF strings33,39,43-45 and is accompanied by a reduction in platelet surface area coverage,46,47 aggregate volume,40 and channel occlusion.26 Similar results were obtained upon targeting the A1 domain of VWF,39,43 in the presence of VWF-free plasma26 or upon desmopressin treatment of the endothelium.48 This fits with the textbook knowledge that the contribution of platelet adhesion to immobilized VWF via GPIb-V-IX is considerably accelerated by high shear forces present in the arterial circulation, as a consequence of conformational changes in the immobilized VWF. Shear rate (per second) and shear stress (in dynes per square centimeter) are the 2 shear variables that are used in flow chamber studies. Shear rate is the rate at which adjacent layers of the blood are moving with respect to each other and increases while approaching the vessel wall. As platelet adhesion occurs at the vessel wall, wall shear rate is meant in this review when talking about shear rate. Shear stress is the (viscous) force of the blood applied on the vessel wall and is a linear function of the wall shear rate and the blood viscosity. For the major vessels of the human circulatory system, the wall shear rate can be compared with the shear stress (Figure 3). The variety in shear between the vessels is partly dependent on the diameter of the vessel (ie, smaller blood vessels have a higher shear rate/stress than bigger blood vessels). An area for future research is to investigate whether platelets have a shear optimum when adhering to the endothelium and, if so, at which wall shear rate this optimum occurs.
Schematic overview of the various interactions between platelet and endothelial surface molecules. Platelets interact with activated ECs via the following main mechanisms: (1) Platelet rolling via interaction of platelet GPIb-IX-V with endothelial-released VWF with a supporting role for the P-selectin/PSGL-1 axis. (2) Firm platelet adhesion to the endothelium via interaction of platelet αIIbβ3 with endothelial αvβ3 (via VWF, fibrinogen, fibronectin) and ICAM-1 (via fibrinogen). Alternatively, platelet αIIbβ3 can also interact with the endothelial GPIb-IX-V (via VWF). (3) A stimulatory role for thrombin and the thrombospondin-1 (TSP-1)/CD36 axis in platelet activation. More detailed information is provided in the text.
Schematic overview of the various interactions between platelet and endothelial surface molecules. Platelets interact with activated ECs via the following main mechanisms: (1) Platelet rolling via interaction of platelet GPIb-IX-V with endothelial-released VWF with a supporting role for the P-selectin/PSGL-1 axis. (2) Firm platelet adhesion to the endothelium via interaction of platelet αIIbβ3 with endothelial αvβ3 (via VWF, fibrinogen, fibronectin) and ICAM-1 (via fibrinogen). Alternatively, platelet αIIbβ3 can also interact with the endothelial GPIb-IX-V (via VWF). (3) A stimulatory role for thrombin and the thrombospondin-1 (TSP-1)/CD36 axis in platelet activation. More detailed information is provided in the text.
Wall shear rates and corresponding shear stresses in the vascular system. The data presented are from de Groot and Sixma83 and Sakariassen et al84 assuming blood as a Newtonian fluid with a constant viscosity and a laminar flow. Asterisk indicates that shear stress is irrelevant because of microcirculatory blood flow.
Wall shear rates and corresponding shear stresses in the vascular system. The data presented are from de Groot and Sixma83 and Sakariassen et al84 assuming blood as a Newtonian fluid with a constant viscosity and a laminar flow. Asterisk indicates that shear stress is irrelevant because of microcirculatory blood flow.
In addition to VWF-GPIb, multiple in vivo and in vitro studies also show a role for both platelet PGSL-1 with endothelial P-selectin46,49 and platelet P-selectin with endothelial PSGL-150 in mediating platelet rolling and adhesion to activated endothelium. Antibodies against anti–P-selectin have been shown to reduce platelet translocation and binding to VWF strings,44 platelet adhesion,51 and surface area covered46 under both low shear (2.5 dynes/cm2) and high shear (50 dynes/cm2, 1500/s) flow conditions (Table 1; supplemental Table 2). Although platelets contain ∼8900 copies of P-selectin per platelet,52 PSGL-1 was shown to be only present in small amounts on the platelet protein level.49 Burkhart et al52 even failed to detect PGSL-1 in the human platelet proteome. Yet, the latter finding could be influenced by the fact that PSGL-1 is heavily glycosylated, which potentially hampers its digestion by trypsin, and hence the subsequent detection by mass spectrometry. PSGL-1 is functionally expressed, albeit at low levels, by ECs in certain organs under conditions of chronic inflammation.50,53 Furthermore, it has been suggested that activated platelets might also use the GPIb-IX-V complex as an alternative receptor for rolling over endothelial P-selectin.33 Yet, to our knowledge, the latter study by Romo et al received no follow up in literature so far, and a binding site for P-selectin on GPIb has not been reported. Moreover, inhibition of GPIb or P-selectin did not result in a complete inhibition of the adhesive potential of P-selectin or GPIb-expressing Chinese hamster ovary cells.33 Taken together, this points to a supporting role of the P-selectin/PSGL-1 axis in platelet-endothelium interaction, in particular under conditions where little VWF is present.
Mechanisms of platelet adhesion, activation, and thrombus formation on activated endothelium
Stable adhesion of platelets to the endothelium requires activation of integrins on the platelet and endothelial surface. A key player herein is platelet αIIbβ3 integrin (Table 1).54 Its contribution to platelet adhesion and thrombus formation on activated endothelium was examined using a range of inhibitors that compete with the binding of VWF and fibrinogen to the integrin, often by (in)directly interacting with the RGD binding pockets of αIIbβ3.55 Similar to blockage of GPIbα, αIIbβ3 inhibition resulted in reduced platelet-containing VWF strings44 accompanied by a reduction in platelet adhesion56 and hence reduced platelet surface area coverage,25,34,46,47,57-59 platelet aggregate volume,40 and channel occlusion26,60 (Table 1). Integrin αIIbβ3 interacts with the endothelium via the bridging molecules VWF, fibrinogen, and fibronectin, which can all bind to endothelial αvβ3 integrin. Inhibition of endothelial αvβ3 reduces stable platelet adhesion by ∼50%, both under flowing61 and static conditions,62,63 suggesting the involvement of other receptors in mediation stable platelet-endothelial interactions. Indeed, endothelial ICAM-1, using fibrinogen as a bridging molecule, and endothelial GPIb-IX-V, via VWF, have also been shown to interact with platelet αIIbβ3 and mediate platelet adhesion (Figure 2).62
Strikingly, the contribution of platelet receptors in the subsequent thrombus forming process has only investigated in a limited manner. So far, interventions into soluble platelet activation factors revealed the importance of the coagulation cascade, primarily via thrombin,24-26 but also of secondary feedback loops via cyclooxygenase 126 and of adenosine triphosphate-P2X1 interaction64 in mediating thrombus formation on activated endothelium. In addition, there appears to be a role for the TSP-1–CD36/CD47 axis in mediating platelet arrest.65 Lagadec et al65 hypothesized that the interaction of TSP-1 with CD36 induces the exposition of the cell-binding domain of TSP-1, allowing its ligation with platelet CD47 and subsequent activation of platelet αIIbβ3 integrin. This fits with in vitro flow chamber and in vivo arterial thrombosis studies, which indicate a platelet activating and thrombus-stabilizing role for TSP-1, via CD36.66,67 Moreover, TSP-1 has also been shown to protect endothelium-bound VWF from ADAMTS13-mediated degradation, thereby further enhancing the dynamic recruitment of platelets into developing thrombi.68 The observation that the TSP-1/CD36 interaction may also stimulate platelet activation by inhibition of the inhibitory cyclic adenosine monophosphate/cyclic guanosine monophosphate signaling pathways in platelets,69,70 and that TSP-1 is present in high amounts in the α-granules of platelets (±100 000 copies/platelet),52 makes TSP-1 potentially a vital player in platelet activation and stable thrombus formation on inflamed endothelium.
Platelet interactions with ECs from lymphatic or xenogeneic origin
Beyond their well-known role as mediators of hemostasis and thrombosis, platelets are also involved in mouse embryonic lymphatic development. Intriguingly, platelet adhesion and activation to lymphatic ECs is regulated via a different mechanism to that in vascular ECs. Podoplanin, constitutively expressed by LECs but not present in vascular ECs, supports platelet arrest to LECs via interaction with platelet CLEC-2.71 The CLEC-2 receptor signals via a similar signaling pathway as GPVI,7 and inhibition of their downstream kinases Src and Syk reduces stable platelet-LEC interactions and platelet surface area coverage.71 Interestingly, although blockage of integrin αIIbβ3 did abolish aggregate formation on LECs, it also led to an increase in platelet surface area coverage. This latter is likely because of the fact that CLEC-2 levels in human platelets are much lower than those for αIIbβ3 (2000 vs 64 200–83 300 per platelet, respectively),52 and platelets thus preferentially bind to activated αIIbβ3, and form compact 3D aggregates, on LECs. Yet, upon blockage of αIIbβ3, platelets only adhere to podoplanin and form a homogeneous monolayer, which results in an increased surface area coverage. This example highlights that merely taking output parameters into account does not always adequately reflect the actual underlying process.
Endothelial-lined flow chamber studies have also been used as a model for xenograft rejection, showing an essential role for the complement system in platelet interaction with ECs of xenogeneic origin.23,25,46 Whether perfusion of human blood over allogeneic ECs (ie, from different individuals of the same species) also induces some activation of the complement system or not remains to be established. Briefly, Galbusera et al46 found that human serum induced porcine EC activation and thrombosis in a parallel plate flow chamber. The underlying mechanism involved complement-induced endothelial activation, with reactive oxygen species–triggered overexpression of endothelial-expressed P-selectin and αvβ3 as a consequence (supplemental Table 2). This proposed mechanism was confirmed by Rataj et al,23 who demonstrated that blockage of complement factor 5 reduced platelet surface area coverage. In addition, Harris et al25 recently showed that galactose 1,3α-galactose transferase–deficient porcine ECs resist hyperacute rejection and display a significant decrease in human platelet surface area coverage and aggregate volume when compared with wild-type porcine ECs. Combined, these findings from flow chamber studies on xenoactivation could be useful to assist with finding new strategies to control platelet activation and prevent xenograft rejection. This is relevant given the interest in xenotransplantation of pig organs into humans as a possible strategy to solve the current shortage of organs for human transplant.
Conclusion and future perspectives
Over recent years, technical advances have led to the development of a range of endothelial-lined flow chamber devices. In this review, we show that these devices hold promise in the field of hemostasis to provide insight into the main players in the cross talk between platelets, the coagulation system, leukocytes, and the endothelium. Although there is ample insight into the mechanisms underlying platelet rolling and arrest on activated endothelium (Figure 2), it remains obscure what happens after platelet adhesion. So far, the importance of the coagulation cascade, primarily via thrombin, and secondary feedback loops, among others via TSP-1, in mediating thrombus formation and stabilization on activated endothelium have been revealed. Of thrombi formed on collagen type I, it has been demonstrated that they consist of at least 3 platelet subpopulations: aggregating platelets with (reversible) integrin activation, procoagulant platelets exposing phosphatidylserine and binding coagulation factors, and contracting platelets with cell-cell contacts residing in the core of the thrombus.72,73 An open question is if, and to what extent, thrombus composition on activated endothelium differs from thrombus formation on collagen and between ECs from different vascular beds. Herein, an important technical challenge lies in including the coagulation system in endothelial-lined flow chamber studies in a controlled way. The study by Ciciliano et al26 is a solid attempt at this in recent years.
Recent technological advances in the flow chamber field partly stem from the high flexibility in design of microfluidics and have, among others, resulted in microfluidics that model the microvasculature60,74 or stenotic arteries.75,76 A multiparameter-based analysis of flow chamber experiments is another recent development.77 Overall, the different designs, setups, and analyses between flow chamber studies do not necessarily have to hamper comparison of experimental results, as long as key variables, such as flow channel dimensions, EC origin plus passage number, presence/absence of RBCs, and/or divalent cations Ca2+ and Mg2+ are routinely reported. Despite a good correlation between the experimental outcome of in vitro flow chamber experiments and in vivo arterial thrombosis studies,6 it is important to be aware of the characteristics of each model. For instance, the CLEC-2 ligand podoplanin is not present in vascular ECs but does become upregulated in the wall of the inferior vena cava during a murine model of deep vein thrombosis, modulating thrombosis.78 In a vascular endothelial-lined flow chamber model this prothrombotic role of CLEC-2 would not be observed. Vice versa, flow chamber studies can provide insight into in vivo studies, for instance in the underlying mechanism of how FeCl3, a commonly used trigger of vascular damage, induces thrombosis.26
Mechanistically, a rapidly emerging and in vogue topic is on the interactions of platelet-derived microparticles with the (activated) endothelium and the short- and long-term cellular effects of these interactions.79 In addition, studies into the role of the glycocalyx in platelet-endothelial interactions,80 postthrombotic processes such as fibroblast infiltration into the thrombus,81 and platelet-dependent matrix degradation82 provide promising avenues to provide novel mechanistic insight.
In sum, in the short-term endothelial-lined flow chamber studies are suited to increase our knowledge of the mechanisms underlying platelet activation and thrombus formation on activated endothelium. In the long term, they hold promise for opening up new investigatory paths to find targets that are only involved in the interplay of vascular cells and hemostatic cells in pathological chronic conditions such as diabetes and atherosclerosis.
The online version of this article contains a data supplement.
Acknowledgments
The authors are grateful to R. Koenen (Maastricht University) and M. T. Harper (Cambridge University, Cambridge, United Kingdom) for providing valuable suggestions and critical reading of the manuscript.
This work was supported by grants from the Dutch Heart Foundation (2015T79) (D.M.C., T.G.M., and J.M.E.M.C.) and the Netherlands Organization for Scientific Research (NWO Vidi 016.156.421) (J.M.E.M.C.).
Authorship
Contribution: D.M.C., T.G.M., J.M.E.M.C. analyzed literature and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judith M. E. M. Cosemans, Maastricht University, Universiteitssingel 50, 6200 MD Maastricht, The Netherlands; e-mail: judith.cosemans@maastrichtuniversity.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal