Key Points
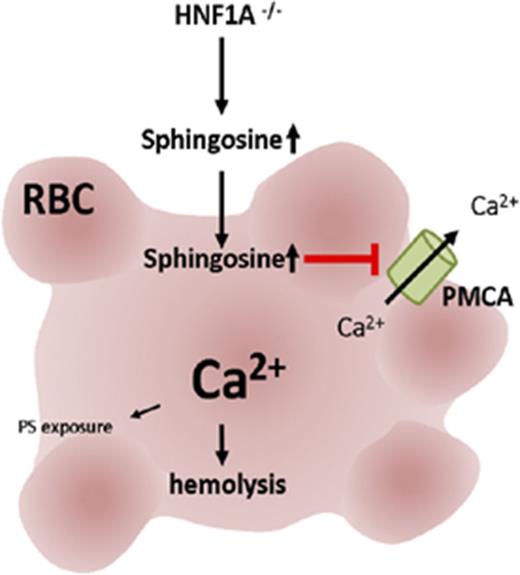
HNF1A deficiency in mice leads to non–cell-autonomous anemia caused by altered red blood cell (RBC) sphingolipids leading to hemolysis.
Sphingosine loading of WT RBCs phenocopies osmotic fragility and high calcium of HNF1A−/− RBCs due to suppressed plasma-membrane Ca2+-ATPase.
Abstract
The hepatocyte nuclear factor (HNF) family regulates complex networks of metabolism and organ development. Human mutations in its prototypical member HNF1A cause maturity-onset diabetes of the young (MODY) type 3. In this study, we identified an important role for HNF1A in the preservation of erythrocyte membrane integrity, calcium homeostasis, and osmotic resistance through an as-yet unrecognized link of HNF1A to sphingolipid homeostasis. HNF1A−/− mice displayed microcytic hypochromic anemia with reticulocytosis that was partially compensated by avid extramedullary erythropoiesis at all erythroid stages in the spleen thereby excluding erythroid differentiation defects. Morphologically, HNF1A−/− erythrocytes resembled acanthocytes and displayed increased phosphatidylserine exposure, high intracellular calcium, and elevated osmotic fragility. Sphingolipidome analysis by mass spectrometry revealed substantial and tissue-specific sphingolipid disturbances in several tissues including erythrocytes with the accumulation of sphingosine as the most prominent common feature. All HNF1A−/− erythrocyte defects could be simulated by exposure of wild-type (WT) erythrocytes to sphingosine in vitro and attributed in part to sphingosine-induced suppression of the plasma-membrane Ca2+-ATPase activity. Bone marrow transplantation rescued the anemia phenotype in vivo, whereas incubation with HNF1A−/− plasma increased the osmotic fragility of WT erythrocytes in vitro. Our data suggest a non–cell-autonomous erythrocyte defect secondary to the sphingolipid changes caused by HNF1A deficiency. Transcriptional analysis revealed 4 important genes involved in sphingolipid metabolism to be deregulated in HNF1A deficiency: Ormdl1, sphingosine kinase-2, neutral ceramidase, and ceramide synthase-5. The considerable erythrocyte defects in murine HNF1A deficiency encourage clinical studies to explore the hematological consequences of HNF1A deficiency in human MODY3 patients.
Introduction
The hepatocyte nuclear factor (HNF) family of transcription factors regulates organ development and numerous metabolic pathways in glucose, amino acid, and lipid homeostasis.1 HNFs control the transcription of hundreds of genes in complex networks where family members act separately or together.1 The prototypical member of the family HNF1A regulates lipid and carbohydrate metabolism in hepatocytes1 and pancreatic island cells2 and kidney function.3 Human loss-of-function mutations in HNF1A and HNF4A cause type 3 and type 1, respectively, of maturity-onset diabetes of the young (MODY): an inherited autosomal-dominant genetic disorder of pancreatic β cells characterized by the onset of diabetes mellitus before the age of 25.4-6 A mutation in HNF1B causes a syndrome of diabetes-related extrapancreatic features with kidney disease called MODY5.7 Mechanistically, HNF1A is involved in the expression of GLUT1 and GLUT2 in pancreatic β cells and angiotensin-converting enzyme 2 in pancreatic islets.8,9 In humans, the HNF1A gene produces 3 alternate isomers, whereas rodents express only HNF1A(A).10 Deletion of HNF1A in mice results in a phenotype characterized by progressive hepatic dysfunction, phenylketonuria, renal Fanconi syndrome,3 diabetes mellitus, and growth hormone insensitivity.11
In addition to their metabolic functions,1,12 HNFs such as HNF1B, HNF3, HNF4, and HNF6 regulate organogenesis, cell differentiation, and tumor growth.12-17 Two family members have been linked to lymphopoiesis: HNF1A as cell-intrinsic factor in adult B lymphopoiesis18 and hepatic HNF6 as a non–cell-intrinsic factor in fetal B lymphopoiesis.19 Evidence of HNF involvement in red blood cell (RBC) biology is scarce with the exception of HNF4 participating in the regulation of erythropoietin gene transcription in Hep3B cells20 and 2 case reports on MODY3 patients where anemia was mentioned as part of clinical history.21,22 During our studies in HNF1A-deficient mice,18 we noticed abnormalities in RBCs that we followed here to identify an important role for HNF1A in the preservation of RBC membrane integrity, osmotic resistance, and calcium homeostasis through an as-yet unrecognized impact on sphingolipid metabolism.
Materials and methods
Mice, hematology, and flow cytometry
HNF1A+/+ and HNF1A−/− mice were on a 129 Svj × C57BL/6J background.3 Whole-blood parameters were determined with a scil VetABC Analyzer, and blood smears stained with Accustain Wright-Giemsa (Sigma-Aldrich). Reticulocytes were measured using Retic-Count (BD Biosciences) followed by Ter119 staining. The following antibodies/reagents were used: CD11b, CD11c, CD49b, CD3ε, Ter119 (Miltenyi Biotec), B220, CD8a, CD4 (eBioscience), CD71, Gr-1, CD16/32, CD45.2, Sca-1, c-Kit (BD Biosciences), CD127 (AbDSerotec), fluorescein isothiocyanate–annexin V (Beckman Coulter). Erythroid precursors were isolated by depletion with CD11b-phycoerythrin (PE), CD3ε-PE, CD45R(B220)-PE, and anti-PE MicroBeads and subsequent anti-CD71-allophycocyanin/anti-allophycocyanin MicroBeads magnetic separation (Miltenyi Biotec).
Osmotic fragility, Fluo-4/AM, and Fura Red/AM measurements
Osmotic fragility was measured as described23 with or without preincubation with sphingosine (Sigma-Aldrich), C16-ceramide (Matreya LLC), vanadate, aurin–tricarboxylic acid (Sigma-Aldrich), or vehicle. RBCs were labeled with Fluo-4/AM (Molecular Probes) in Ringer containing 1 mM CaCl2 and 2 µM Fluo-4/AM for 45 minutes and treated or not with sphingosine for 30 minutes at room temperature. In some experiments, RBCs were stimulated with 1 µM ionomycin (Enzo Life Sciences) 2 minutes prior to analysis or a time course was performed without and with 0.0125 µM ionomycin. Fura Red assays were performed with modifications as described in human leukocytes24 with 5 µM Fura Red/AM incubation for 40 minutes at 37°C. Ratiometric analysis was performed using the Violet and Blue lasers and calculating emission ratio at 405 nm/488 nm. 5(6)-Carboxyeosin (10 µM; Enzo Life Sciences) or dimethyl sulfoxide (DMSO) (0.1%) was added for 30 minutes at room temperature followed by addition or not of 0.05 µM ionomycin. For incubation of RBCs with plasma (1:1), heparin was used (see supplemental Material and methods [available on the Blood Web site] for detailed description).
Immunoblotting and real-time PCR
RBC ghost proteins were immunoblotted with antibodies against plasma-membrane Ca2+-ATPase 1/4 (PMCA1/4), glycophorin A (Santa Cruz Biotechnology), band 3 (Cell Signaling Technology), and β-actin (Sigma-Aldrich). RNA was isolated using the InviTrap Spin Universal RNA Minikit (Invitek), and complementary DNA (cDNA) was generated with the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific). Real-time polymerase chain reaction (PCR) was performed using SYBR Green in a Bio-Rad CFX cycler.
Bone marrow transplantation and sphingolipid measurements
Statistics
Analysis was performed using 1-way analysis of variance (ANOVA) followed by the Tukey multiple comparison test, 2-way ANOVA followed by the Bonferroni post hoc test, or the Student t test.
Detailed information is provided in supplemental Material and methods.
Results
HNF1A−/− mice display hypochromic microcytic anemia
Analysis of peripheral blood from HNF1A−/− mice showed a decrease in hematocrit (HCT) and hemoglobin (HGB) compared with wild type (WT) (Table 1). The MCV and MCH were reduced in HNF1A−/− RBCs, whereas RDW was increased and MCHC remained unchanged (Table 1). Total RBC counts including reticulocytes were apparently not altered but flow cytometric analysis using Ter119 and thiazole orange revealed a threefold to fourfold increase of reticulocytes in HNF1A−/− mice (Figure 1A). Accordingly, mature RBCs were decreased after subtraction of reticulocytes (Figure 1B). Heterozygous mice had a significantly decreased HCT compared with controls and higher HGB, MCV, and RDW compared with knockouts (KOs), respectively (Table 1). Platelets were also significantly increased in HNF1A−/− mice compared with controls (714 000/µL blood ± 98 000/µL blood vs 312 000/µL blood ± 50 000/µL blood, n = 3 each, P = .01). No changes in serum iron or erythropoietin were observed in HNF1A−/− compared with controls (2.7 ng/µL ± 0.6 ng/µL vs 2.6 ng/µL ± 0.5 ng/µL, n = 3, P = .9, and 89.6 pg/mL ± 20.0 pg/mL vs 133.9 pg/mL ± 32.9 pg/mL, n = 3, P = .35). These observations were consistent with the presence of hypochromic microcytic anemia with normal iron and erythropoietin and accompanied by reticulocytosis.
Characteristics of peripheral blood of HNF1A+/+, HNF1A+/−, and HNF1A−/− mice
| Genotype . | HNF1A+/+, n = 7 . | HNF1A+/−, n = 7 . | HNF1A−/−, n = 7 . |
|---|---|---|---|
| HCT, % | 57.4 ± 1.5 | 50.0 ± 1.1††† | 48.3 ± 0.9*** |
| HGB, g/dL | 16.7 ± 0.5 | 16.2 ± 0.4 | 14.0 ± 0.3§§,*** |
| RBC, (×106)/µL blood (including reticulocytes) | 10.7 ± 0.2 | 11.1 ± 0.4 | 10.4 ± 0.3 |
| MCV, µm3 | 53.7 ± 0.5 | 51.6 ± 1.2 | 46.7 ± 1.2§,** |
| MCH, pg | 15.7 ± 0.2 | 14.6 ± 0.4 | 13.6 ± 0.4** |
| MCHC, g/dL | 29.1 ± 0.3 | 28.3 ± 0.3 | 29.0 ± 0.4 |
| RDW, % | 11.5 ± 0.2 | 12.0 ± 0.2 | 15.6 ± 0.3§§§,*** |
| Genotype . | HNF1A+/+, n = 7 . | HNF1A+/−, n = 7 . | HNF1A−/−, n = 7 . |
|---|---|---|---|
| HCT, % | 57.4 ± 1.5 | 50.0 ± 1.1††† | 48.3 ± 0.9*** |
| HGB, g/dL | 16.7 ± 0.5 | 16.2 ± 0.4 | 14.0 ± 0.3§§,*** |
| RBC, (×106)/µL blood (including reticulocytes) | 10.7 ± 0.2 | 11.1 ± 0.4 | 10.4 ± 0.3 |
| MCV, µm3 | 53.7 ± 0.5 | 51.6 ± 1.2 | 46.7 ± 1.2§,** |
| MCH, pg | 15.7 ± 0.2 | 14.6 ± 0.4 | 13.6 ± 0.4** |
| MCHC, g/dL | 29.1 ± 0.3 | 28.3 ± 0.3 | 29.0 ± 0.4 |
| RDW, % | 11.5 ± 0.2 | 12.0 ± 0.2 | 15.6 ± 0.3§§§,*** |
Data are presented as mean ± standard error of the mean (SEM). Age and sex were for HNF1A+/+ (161 ± 31 days; female [n = 5], male [n = 2]), HNF1A+/− (158 ± 24 days; female [n = 3], male [n = 4]), and HNF1A−/− mice (164 ± 31 days; female [n = 5], male [n = 2]).
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration; RDW, red cell distribution width.
P < .01 and ***P < .001 for HNF1A+/+ vs HNF1A−/−, †††P < .001 for HNF1A+/+ vs HNF1A+/−, and §P < .05, §§P < .01, and §§§P < .001 for HNF1A+/− vs HNF1A−/− (1-way ANOVA followed by Tukey post hoc analysis).
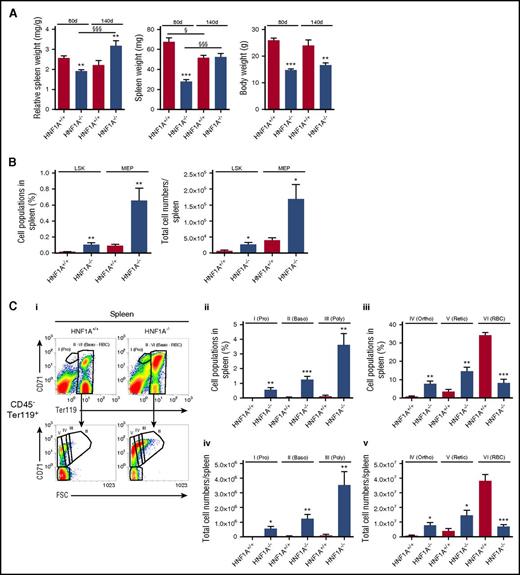
Anemia, reticulocytosis, and analysis of hematopoietic stem cells, MEPs, and erythroid cell populations in the bone marrow of HNF1A−/− mice. (A) Flow cytometric reticulocyte quantification using thiazole orange in HNF1A+/+ and HNF1A−/− mice (n = 7 each). Representative images of Ter119+Thiazole orange+ reticulocytes (i), quantification of reticulocytes in percentage (ii) and total numbers per microliter of whole blood (iii). (B) Calculation of mature RBC counts per microliter of blood as performed by subtraction of reticulocyte counts from total RBC counts. (C) Representative images and gating strategy for LSK (Lin−Sca-1+c-Kit+IL-7R−) cells and MEPs (Lineage−IL-7R−Sca-1−c-Kit+CD34−CD16/32−/low [FcγRII/III]) in bone marrow from HNF1A−/− and HNF1A+/+ mice (n = 6 each) as analyzed by flow cytometry (i). LSK and MEP cell populations were determined according to Kondo et al,26 Pronk et al,27 and Murphy et al28 after eliminating lineage-positive cells (Lin−: CD11b, Gr-1, CD49b, CD11c, CD3ε, CD4, CD8, B220, Ter119). Percentage (ii) and total cell numbers per femur length (iii) of LSKs and MEPs as provided. Cell counts per femur were divided by femur length to normalize for the shorter HNF1A−/− femura. (D) Representative fluorescence-activated cell sorter (FACS) plots and gating strategy of erythroid cell populations by combination of Ter119 and CD71 expression and determination of cell size in regions I to VI, respectively, as described.29,30 Erythroid populations were distinguished in early proerythroblasts (Pro; Ter119medFSChighCD71high), basophilic erythroblasts (Baso; Ter119highFSChighCD71high), polychromatic erythroblasts (Poly; Ter119highFSCmed/highCD71med/high), orthochromatic erythroblasts (Ortho; Ter119highFSCmedCD71med), reticulocytes (Retic; Ter119highFSClowCD71low), and mature RBCs (Ter119highFSClowCD71−) (i). Total cell numbers of proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts (ii), orthochromatic erythroblasts, reticulocytes, and mature RBCs (iii) per femur normalized for femur length are provided as indicated. All data are presented as mean ± SEM. *P < .05, **P < .01, and ***P < .001 for HNF1A+/+ vs HNF1A−/− (Student t test).
Anemia, reticulocytosis, and analysis of hematopoietic stem cells, MEPs, and erythroid cell populations in the bone marrow of HNF1A−/− mice. (A) Flow cytometric reticulocyte quantification using thiazole orange in HNF1A+/+ and HNF1A−/− mice (n = 7 each). Representative images of Ter119+Thiazole orange+ reticulocytes (i), quantification of reticulocytes in percentage (ii) and total numbers per microliter of whole blood (iii). (B) Calculation of mature RBC counts per microliter of blood as performed by subtraction of reticulocyte counts from total RBC counts. (C) Representative images and gating strategy for LSK (Lin−Sca-1+c-Kit+IL-7R−) cells and MEPs (Lineage−IL-7R−Sca-1−c-Kit+CD34−CD16/32−/low [FcγRII/III]) in bone marrow from HNF1A−/− and HNF1A+/+ mice (n = 6 each) as analyzed by flow cytometry (i). LSK and MEP cell populations were determined according to Kondo et al,26 Pronk et al,27 and Murphy et al28 after eliminating lineage-positive cells (Lin−: CD11b, Gr-1, CD49b, CD11c, CD3ε, CD4, CD8, B220, Ter119). Percentage (ii) and total cell numbers per femur length (iii) of LSKs and MEPs as provided. Cell counts per femur were divided by femur length to normalize for the shorter HNF1A−/− femura. (D) Representative fluorescence-activated cell sorter (FACS) plots and gating strategy of erythroid cell populations by combination of Ter119 and CD71 expression and determination of cell size in regions I to VI, respectively, as described.29,30 Erythroid populations were distinguished in early proerythroblasts (Pro; Ter119medFSChighCD71high), basophilic erythroblasts (Baso; Ter119highFSChighCD71high), polychromatic erythroblasts (Poly; Ter119highFSCmed/highCD71med/high), orthochromatic erythroblasts (Ortho; Ter119highFSCmedCD71med), reticulocytes (Retic; Ter119highFSClowCD71low), and mature RBCs (Ter119highFSClowCD71−) (i). Total cell numbers of proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts (ii), orthochromatic erythroblasts, reticulocytes, and mature RBCs (iii) per femur normalized for femur length are provided as indicated. All data are presented as mean ± SEM. *P < .05, **P < .01, and ***P < .001 for HNF1A+/+ vs HNF1A−/− (Student t test).
HNF1A−/− mice exhibit efficient stimulation of extramedullary hematopoiesis in the spleen
We then analyzed hematopoiesis in bone marrow and spleens from HNF1A−/− and WT mice. To assess early stages of hematopoiesis, Lineage−IL-7R−Sca-1+c-Kit+ (LSK) cells and megakaryocyte–erythroid progenitor (MEP) cells (Lineage−IL-7R−Sca-1−c-Kit+CD34−CD16/32−/low[FcγRII/III]) were determined (Figure 1C) as described.26-28 Erythroblasts were identified by a combination of Ter119 and CD71 and cell size in region I-VI,29,30 allowing assessment of early proerythroblasts (Pro; Ter119medforward scatter [FSC]highCD71high), basophilic (Baso; Ter119highFSChighCD71high), polychromatic (Poly; Ter119highFSCmed/highCD71med/high), and orthochromatic erythroblasts (Ortho; Ter119highFSCmedCD71med), reticulocytes (Retic; Ter119highFSClowCD71low), and mature RBCs (Ter119highFSClowCD71−) (Figure 1D). Using this approach, we observed that LSKs were increased in the bone marrow of HNF1A−/− mice both in percentage and total numbers, whereas MEPs were higher in percentage but not in total counts (Figure 1C). Of all erythroid differentiation stages, only the early proerythroblasts were approximately twofold increased, whereas neither the basophilic nor polychromatic or orthochromatic erythroblast populations, nor reticulocytes, were altered (Figure 1D).
In many cases of anemia in the mouse, compensatory erythropoiesis takes place in the spleen rather than the bone marrow prompting us to analyze spleens of HNF1A−/− and WT mice for signs of extramedullary erythropoiesis. Whereas the relative spleen weights were reduced in 80-day-old HNF1A−/− mice (published before because of defective B-cell lymphopoiesis18 ), spleen weights increased with age (30% higher than controls after 140 days), suggesting that extramedullary erythropoiesis may be increasingly taking place with time (Figure 2A). Indeed, flow cytometric analysis revealed a fourfold increase of LSKs and MEPs in HNF1A−/− spleens both in percentage and total cell numbers compared with controls (Figure 2B). Furthermore, there was a dramatic rise in all erythroid differentiation stages in HNF1A−/− spleens compared with controls: proerythroblast numbers were elevated a striking 54-fold, basophilic erythroblasts 35-fold, polychromatic erythroblasts 36-fold, orthochromatic erythroblasts 10-fold, and reticulocytes fourfold, respectively (Figure 2C).
Extramedullary erythropoiesis in spleens of HNF1A−/−mice. (A) Relative spleen weight (left), total spleen weight (middle), and body weight (right) of 80-day-old and 140-day-old HNF1A+/+ and HNF1A−/− mice (n = 9 vs 12, and n = 5 vs 6, respectively). **P < .01 and ***P < .001 for 80-day-old or 140-day-old HNF1A+/+ vs HNF1A−/−; §P < .05 and §§§P < .001 for 80-day-old vs 140-day-old HNF1A+/+ or for 80-day-old vs 140-day-old HNF1A−/− (1-way ANOVA followed by Tukey post hoc analysis). (B-C) Splenic cell populations from HNF1A+/+ (n = 5) and HNF1A−/− (n = 6) mice with a mean age of 140 days as analyzed by flow cytometry. (B) The percentage (left) and total cell numbers (right) corresponding to LSK and MEP cell populations as indicated. (C) Representative images and gating strategy of HNF1A+/+ and HNF1A−/− erythroid populations in spleen (i). The percentage (ii-iii) and total cell numbers (iv-v) in spleen corresponding to proerythroblasts (Pro), basophilic erythroblasts (Baso), polychromatic erythroblasts (Poly), orthochromatic erythroblasts (Ortho), reticulocytes (Retic), and mature RBCs as indicated. *P < .05, **P < .01, and ***P < .001 for HNF1A+/+ vs HNF1A−/− (Student t test).
Extramedullary erythropoiesis in spleens of HNF1A−/−mice. (A) Relative spleen weight (left), total spleen weight (middle), and body weight (right) of 80-day-old and 140-day-old HNF1A+/+ and HNF1A−/− mice (n = 9 vs 12, and n = 5 vs 6, respectively). **P < .01 and ***P < .001 for 80-day-old or 140-day-old HNF1A+/+ vs HNF1A−/−; §P < .05 and §§§P < .001 for 80-day-old vs 140-day-old HNF1A+/+ or for 80-day-old vs 140-day-old HNF1A−/− (1-way ANOVA followed by Tukey post hoc analysis). (B-C) Splenic cell populations from HNF1A+/+ (n = 5) and HNF1A−/− (n = 6) mice with a mean age of 140 days as analyzed by flow cytometry. (B) The percentage (left) and total cell numbers (right) corresponding to LSK and MEP cell populations as indicated. (C) Representative images and gating strategy of HNF1A+/+ and HNF1A−/− erythroid populations in spleen (i). The percentage (ii-iii) and total cell numbers (iv-v) in spleen corresponding to proerythroblasts (Pro), basophilic erythroblasts (Baso), polychromatic erythroblasts (Poly), orthochromatic erythroblasts (Ortho), reticulocytes (Retic), and mature RBCs as indicated. *P < .05, **P < .01, and ***P < .001 for HNF1A+/+ vs HNF1A−/− (Student t test).
HNF1A−/− RBCs feature altered morphology on Wright-Giemsa staining
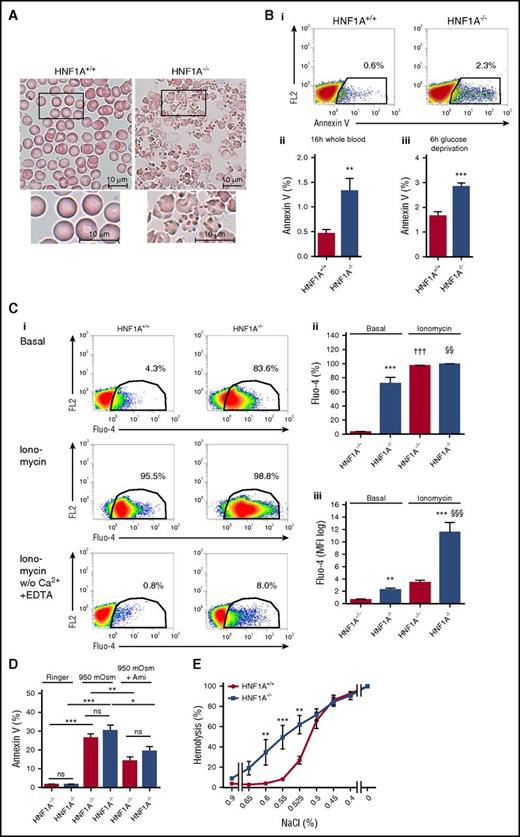
The increased turnover of RBCs in HNF1A−/− mice may indicate reduced RBC lifespan due to altered morphology. Wright-Giemsa staining of peripheral blood smears revealed, indeed, a very characteristic appearance of HNF1A−/− RBCs resembling most closely acanthocytosis without the presence of fragmentocytes, stomatocytes, target cells, spherocytes, or poikilocytes (Figure 3A).
Wright-Giemsa staining, PS exposure, intracellular calcium, sensitivity to hyperosmolarity, and osmotic fragility of HNF1A−/−RBCs. (A) Representative light microscopy images of Wright-Giemsa–stained peripheral blood smears (n = 3; original magnification ×100) of HNF1A+/+ and HNF1A−/− mice. Bars represent 10 μm. (B) Representative images of annexin V flow cytometric analysis of HNF1A+/+ (n = 6) and HNF1A−/− (n = 6) RBCs, 16 hours after collecting peripheral blood samples and incubation at room temperature. The FL2 channel was empty (i). Quantification of the mean percentage of annexin V binding on HNF1A+/+ and HNF1A−/− RBCs (n = 6 each) 16 hours after collecting blood samples (ii) or 6 hours after glucose deprivation of washed RBCs (iii). **P < .01 and ***P < .001 (Student t test). (C) Representative flow cytometric analysis of intracellular calcium content in HNF1A+/+ and HNF1A−/− RBCs prestained with Fluo-4 and incubated in Ringer without (i, top) and with ionomycin (1 µM; i, middle), with ionomycin (1 µM; i, bottom) in Ringer without Ca2+ in the presence of 1 mM EDTA measured after 2 minutes of incubation. The FL2 channel was empty. Quantification of the percentage (ii) and mean fluorescence intensity (MFI) (iii) of HNF1A+/+ (n = 4) and HNF1A−/− (n = 4) RBCs positive for Fluo-4. **P < .01 and ***P < .001 for untreated HNF1A+/+ vs HNF1A−/−, †††P < .001 for treated vs untreated HNF1A+/+ and §§P < .01 and §§§P < .001 for treated vs untreated HNF1A−/− (1-way ANOVA followed by Tukey post hoc analysis). (D) Mean percentage of annexin V binding on HNF1A+/+ and HNF1A−/− RBCs (n = 3 each) 4 hours after incubation with 950 mOsm Ringer solution in the presence or absence of 50 µM amitriptyline (Ami). *P < .05, **P < .01, and ***P < .001 for 950 mOsm vs untreated HNF1A+/+ or vs 950 mOsm plus Ami; for 950 mOsm vs untreated HNF1A−/− or vs 950 mOsm plus Ami as indicated (1-way ANOVA followed by Tukey post hoc analysis). (E) Osmotic fragility in RBCs from HNF1A+/+ and HNF1A−/− mice (n = 3 each) using solutions with decreasing concentrations of NaCl. **P < .01 and ***P < .001 for untreated vs treated RBCs (2-way ANOVA followed by Bonferroni post hoc analysis). ns, not significant.
Wright-Giemsa staining, PS exposure, intracellular calcium, sensitivity to hyperosmolarity, and osmotic fragility of HNF1A−/−RBCs. (A) Representative light microscopy images of Wright-Giemsa–stained peripheral blood smears (n = 3; original magnification ×100) of HNF1A+/+ and HNF1A−/− mice. Bars represent 10 μm. (B) Representative images of annexin V flow cytometric analysis of HNF1A+/+ (n = 6) and HNF1A−/− (n = 6) RBCs, 16 hours after collecting peripheral blood samples and incubation at room temperature. The FL2 channel was empty (i). Quantification of the mean percentage of annexin V binding on HNF1A+/+ and HNF1A−/− RBCs (n = 6 each) 16 hours after collecting blood samples (ii) or 6 hours after glucose deprivation of washed RBCs (iii). **P < .01 and ***P < .001 (Student t test). (C) Representative flow cytometric analysis of intracellular calcium content in HNF1A+/+ and HNF1A−/− RBCs prestained with Fluo-4 and incubated in Ringer without (i, top) and with ionomycin (1 µM; i, middle), with ionomycin (1 µM; i, bottom) in Ringer without Ca2+ in the presence of 1 mM EDTA measured after 2 minutes of incubation. The FL2 channel was empty. Quantification of the percentage (ii) and mean fluorescence intensity (MFI) (iii) of HNF1A+/+ (n = 4) and HNF1A−/− (n = 4) RBCs positive for Fluo-4. **P < .01 and ***P < .001 for untreated HNF1A+/+ vs HNF1A−/−, †††P < .001 for treated vs untreated HNF1A+/+ and §§P < .01 and §§§P < .001 for treated vs untreated HNF1A−/− (1-way ANOVA followed by Tukey post hoc analysis). (D) Mean percentage of annexin V binding on HNF1A+/+ and HNF1A−/− RBCs (n = 3 each) 4 hours after incubation with 950 mOsm Ringer solution in the presence or absence of 50 µM amitriptyline (Ami). *P < .05, **P < .01, and ***P < .001 for 950 mOsm vs untreated HNF1A+/+ or vs 950 mOsm plus Ami; for 950 mOsm vs untreated HNF1A−/− or vs 950 mOsm plus Ami as indicated (1-way ANOVA followed by Tukey post hoc analysis). (E) Osmotic fragility in RBCs from HNF1A+/+ and HNF1A−/− mice (n = 3 each) using solutions with decreasing concentrations of NaCl. **P < .01 and ***P < .001 for untreated vs treated RBCs (2-way ANOVA followed by Bonferroni post hoc analysis). ns, not significant.
Enhanced PS exposure and higher intracellular calcium in HNF1A−/− RBCs
RBC membrane alterations are associated with numerous disturbances of homeostasis leading to shortened life span. To address these, we measured phosphatidylserine (PS) exposure in RBCs using annexin V and observed a clear increase in HNF1A−/− RBCs (Figure 3B). This was the case in whole blood and after 6 hours of glucose deprivation in washed RBCs (Figure 3B). Another feature of pathologically altered RBC membrane properties can be disturbances in calcium homeostasis. We thus compared RBC calcium levels between the 2 genotypes by flow cytometry using Fluo-4. We observed that intracellular calcium was dramatically elevated in HNF1A−/− compared with WT RBCs (Figure 3Ci-iii). Furthermore, treatment with ionomycin resulted in a much higher calcium elevation in HNF1A−/− than WT RBCs (Figure 3Ciii). Eliminating calcium and adding EDTA to the extracellular medium greatly reduced the Fluo-4 signal demonstrating the contribution of extracellular calcium (Figure 3Ci bottom). The number of HNF1A−/− RBCs with elevated calcium in response to ionomycin in calcium-free EDTA medium was 10-fold higher than the corresponding number of WT RBCs, possibly indicating elevated calcium in sequestered compartment(s). Also, smaller MCV and MCH might have influenced the fluorescence signal but the effects can be neglected with such robust signals.
Complex changes in the sphingolipidome of HNF1A−/− RBCs with higher ceramide and sphingosine concentrations
Although PS exposure and increased calcium in RBCs can be phenotypic features of various biological processes, some investigators have associated them with RBC shrinkage, cell membrane scrambling, and protease activation to define a process they have termed eryptosis.31-35 Another feature has been the increase of intracellular ceramide generation in response to hyperosmolarity and oxidative stress and its contribution to eryptosis.36,37 We thus used liquid chromatography–tandem mass spectrometry to measure ceramide and numerous other sphingolipids in RBCs and observed several distinctive changes in HNF1A−/− compared with WT RBCs: C16-ceramide, sphingosine, and sphingosine-1-phosphate (S1P) were increased 1.74-fold, 1.9-fold, and 2.7-fold, and sphingomyelin was decreased 0.6-fold, respectively, whereas phosphatidylcholine and lysophosphatidylcholine remained unchanged (Table 2; supplemental Table 1). No differences were observed between WT and heterozygous RBCs (Table 2). Interestingly, the sphingolipidome changes were not only restricted to RBCs but also present in livers and plasma of HNF1A−/− mice albeit with different distinctive patterns (Table 2). In HNF1A−/− livers, sphingosine was increased 1.5-fold, whereas ceramide, S1P, sphingomyelin, and lysophosphatidylcholine were not altered and phosphatidylcholine was decreased 0.6-fold (Table 2). In HNF1A−/− plasma, sphingosine was, again, increased (2.8-fold), whereas S1P, sphingomyelin, and phosphatidylcholine were decreased (0.63-fold, 0.66-fold, and 0.74-fold; Table 2). Cholesterol concentrations were not different between HNF1A+/+ and HNF1A−/− RBCs (2.04 µM ± 0.05 µM vs 2.55 µM ± 0.26 µM, n = 4 each, P = .14).
Sphingolipids in RBCs, plasma, and livers of HNF1A+/+, HNF1A+/−, and HNF1A−/− mice
| Sphingolipids and metabolites . | HNF1A+/+ . | HNF1A+/− . | HNF1A−/− . |
|---|---|---|---|
| Erythrocytes, nM | n = 13 | n = 4 | n = 7 |
| Sphingosine | 5 107 ± 729 | 7 325 ± 324 | 9 691 ± 1 328** |
| C16-ceramide | 364 ± 33 | 438 ± 39 | 633 ± 39*** |
| Lysophosphatidylcholine | 68 985 ± 11 401 | 65 250 ± 8 128 | 237 914 ± 86 273* |
| Sphingomyelin | 112 215 ± 14 145 | 115 150 ± 22 291 | 67 200 ± 8 990* |
| Phosphatidylcholine | 162 477 ± 20 081 | 179 450 ± 31 295 | 139 857 ± 34 142 |
| Plasma, nM | n = 6 | n = 6 | n = 5 |
| Sphingosine | 9 ± 1 | 11 ± 2 | 25 ± 1*** |
| Sphingosine-1-phosphate | 1 233 ± 56 | 1 027 ± 55† | 772 ± 61*** |
| C16-ceramide | 31 ± 5 | 34 ± 3 | 35 ± 8 |
| Lysophosphatidylcholine | 284 564 ± 47 272 | 256 377 ± 40 459 | 377 329 ± 48 852 |
| Sphingomyelin | 58 827 ± 3 096 | 55 587 ± 4 108 | 38 879 ± 4 741** |
| Phosphatidylcholine | 309 929 ± 12 485 | 305 051 ± 12 172 | 228 092 ± 7 580*** |
| Liver, pmol/g | n = 5 | n = 5 | n = 5 |
| Sphingosine | 2 261 ± 266 | 2 064 ± 530 | 3 290 ± 218* |
| Sphingosine-1-phosphate | 19 ± 4 | 21 ± 3 | 37 ± 7 P= .056 |
| C16-ceramide | 16 201 ± 2 509 | 12 977 ± 2 015 | 19 881 ± 1 749 |
| Lysophosphatidylcholine | 153 917 ± 20 759 | 133 929 ± 22 986 | 152 879 ± 41 454 |
| Sphingomyelin | 11 592 ± 2 497 | 10 591 ± 1 115 | 16 831 ± 6 129 |
| Phosphatidylcholine | 166 124 ± 9 639 | 182 145 ± 10 753 | 102 235 ± 19 375* |
| Sphingolipids and metabolites . | HNF1A+/+ . | HNF1A+/− . | HNF1A−/− . |
|---|---|---|---|
| Erythrocytes, nM | n = 13 | n = 4 | n = 7 |
| Sphingosine | 5 107 ± 729 | 7 325 ± 324 | 9 691 ± 1 328** |
| C16-ceramide | 364 ± 33 | 438 ± 39 | 633 ± 39*** |
| Lysophosphatidylcholine | 68 985 ± 11 401 | 65 250 ± 8 128 | 237 914 ± 86 273* |
| Sphingomyelin | 112 215 ± 14 145 | 115 150 ± 22 291 | 67 200 ± 8 990* |
| Phosphatidylcholine | 162 477 ± 20 081 | 179 450 ± 31 295 | 139 857 ± 34 142 |
| Plasma, nM | n = 6 | n = 6 | n = 5 |
| Sphingosine | 9 ± 1 | 11 ± 2 | 25 ± 1*** |
| Sphingosine-1-phosphate | 1 233 ± 56 | 1 027 ± 55† | 772 ± 61*** |
| C16-ceramide | 31 ± 5 | 34 ± 3 | 35 ± 8 |
| Lysophosphatidylcholine | 284 564 ± 47 272 | 256 377 ± 40 459 | 377 329 ± 48 852 |
| Sphingomyelin | 58 827 ± 3 096 | 55 587 ± 4 108 | 38 879 ± 4 741** |
| Phosphatidylcholine | 309 929 ± 12 485 | 305 051 ± 12 172 | 228 092 ± 7 580*** |
| Liver, pmol/g | n = 5 | n = 5 | n = 5 |
| Sphingosine | 2 261 ± 266 | 2 064 ± 530 | 3 290 ± 218* |
| Sphingosine-1-phosphate | 19 ± 4 | 21 ± 3 | 37 ± 7 P= .056 |
| C16-ceramide | 16 201 ± 2 509 | 12 977 ± 2 015 | 19 881 ± 1 749 |
| Lysophosphatidylcholine | 153 917 ± 20 759 | 133 929 ± 22 986 | 152 879 ± 41 454 |
| Sphingomyelin | 11 592 ± 2 497 | 10 591 ± 1 115 | 16 831 ± 6 129 |
| Phosphatidylcholine | 166 124 ± 9 639 | 182 145 ± 10 753 | 102 235 ± 19 375* |
Data are presented as mean ± SEM.
P < .05, **P < .01, ***P < .001 HNF1A+/+ vs HNF1A−/−, and †P < .05 HNF1A+/+ vs HNF1A+/−.
No signs of higher sensitivity to induction of eryptosis in HNF1A−/− RBCs
To test whether high PS, calcium, and ceramide, respectively, were signs of eryptosis in HNF1A−/− RBCs, we tested their susceptibility to hyperosmolaric stress, a stimulus that induces eryptosis including PS exposure in a ceramide- and calcium-dependent manner.38 Surprisingly, HNF1A−/− and WT RBCs were similarly sensitive to 950 mOsm as determined by PS exposure (Figure 3D). The response to hyperosmolarity has been largely attributed to ceramide generation by the acid sphingomyelinase (aSMase), and its inhibition shown to attenuate PS exposure.37,38 Thus, we tested whether HNF1A−/− RBCs that already contained higher ceramide were less susceptible to rescue from hyperosmolarity by the aSMase inhibitor amitriptyline.39 However, we found amitriptyline to be similarly effective in protecting both HNF1A−/− and WT RBCs (Figure 3D). These results suggested that higher PS, calcium, and ceramide in HNF1A−/− RBCs were not signs of eryptosis but, instead, were features of another biological process.
Increased osmotic fragility of HNF1A−/− RBCs
Accordingly, we tested whether the hypochromic microcytic anemia of HNF1A−/− mice along with the characteristic changes in RBC shape, membrane properties, and calcium were consequences of compromised RBC membrane integrity increasing the susceptibility to hemolysis. We thus examined osmotic fragility of HNF1A−/− and WT RBCs and observed that it was, indeed, substantially increased in HNF1A−/− RBCs over the entire range of NaCl concentrations between 0.6% and 0.525% (Figure 3E).
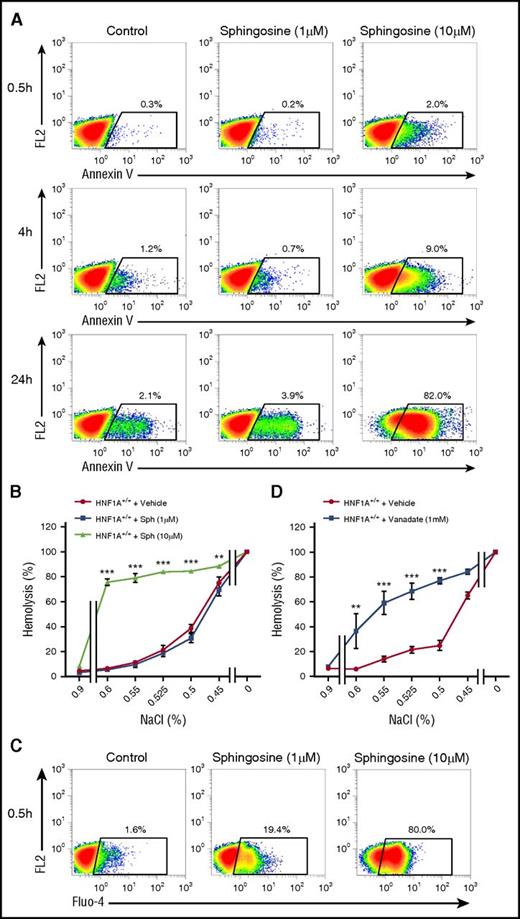
Sphingosine treatment leads to PS exposure, increased osmotic fragility, and higher intracellular calcium in a PMCA-dependent manner
HNF1A−/− RBCs contained twice as much sphingosine as WT RBCs leading us to test whether enrichment of WT RBCs with sphingosine may cause similar changes. To do this, we increased sphingosine concentrations in WT RBCs to the same levels as those contained in HNF1A−/− RBCs by incubating them with different sphingosine concentrations and times and tested the consequences for PS exposure, intracellular calcium, and osmotic fragility. Cells incubated with 1 µM and 10 µM sphingosine featured clearly increased annexin V binding in time- and concentration-dependent manner (Figure 4A). Effective loading was ensured by measuring RBC sphingosine content (12.4 µM ± 0.6 µM in packed cells after 30 minutes of 10 µM sphingosine, n = 3). We then analyzed the osmotic fragility of sphingosine-treated RBCs. Although no signs of hemolysis were present at physiological NaCl concentrations, osmotic fragility was clearly increased for all NaCl concentrations between 0.6% and 0.45% as compared with untreated RBCs (Figure 4B). High intracellular calcium following ionophore treatment has been demonstrated to increase osmotic fragility by a specific calcium-induced lytic vulnerability of the membrane.40 Thus, we measured the effect of sphingosine on intracellular calcium levels by Fluo-4 and flow cytometry. Indeed, intracellular calcium increased in a concentration-dependent manner already 30 minutes after sphingosine treatment (Figure 4C).
Effects of sphingosine on PS exposure, osmotic fragility, and intracellular calcium in WT RBCs. (A) Flow cytometry for annexin V on WT RBCs treated with different sphingosine concentrations (1 µM or 10 µM) or vehicle (ethanol, control) or for several time points as indicated. The FL2 channel was empty. (B) Osmotic fragility in WT RBCs (n = 3) pretreated with different sphingosine (Sph) concentrations or vehicle (ethanol) for 6 hours. (C) FACS plots of WT RBCs preloaded with Fluo-4 and exposed to Ringer with sphingosine (1 µM or 10 µM) or vehicle (ethanol, control) for 30 minutes. The FL2 channel was empty. (D) Osmotic fragility in WT RBCs (n = 3) pretreated with vanadate (1 mM) or vehicle (H2O) for 1 hour at 37°C using solutions with decreasing concentrations of NaCl. **P < .01 and ***P < .001 for untreated vs treated RBCs (2-way ANOVA followed by Bonferroni post hoc analysis for panels B,D).
Effects of sphingosine on PS exposure, osmotic fragility, and intracellular calcium in WT RBCs. (A) Flow cytometry for annexin V on WT RBCs treated with different sphingosine concentrations (1 µM or 10 µM) or vehicle (ethanol, control) or for several time points as indicated. The FL2 channel was empty. (B) Osmotic fragility in WT RBCs (n = 3) pretreated with different sphingosine (Sph) concentrations or vehicle (ethanol) for 6 hours. (C) FACS plots of WT RBCs preloaded with Fluo-4 and exposed to Ringer with sphingosine (1 µM or 10 µM) or vehicle (ethanol, control) for 30 minutes. The FL2 channel was empty. (D) Osmotic fragility in WT RBCs (n = 3) pretreated with vanadate (1 mM) or vehicle (H2O) for 1 hour at 37°C using solutions with decreasing concentrations of NaCl. **P < .01 and ***P < .001 for untreated vs treated RBCs (2-way ANOVA followed by Bonferroni post hoc analysis for panels B,D).
One mechanism by which sphingosine may be potentially increasing calcium and osmotic fragility in RBCs may be through its recently described inhibitory effect on the PMCA, an effect shown in purified enzyme preparations41 but never in intact cells. Short-chain ceramides (C2 and C8) stimulated PMCA activity in the same setup.41 We first used vanadate as a potent nonspecific PMCA inhibitor35 in WT RBCs and observed that osmotic fragility was elevated for the same NaCl concentrations (0.6%-0.5%) as for sphingosine (Figure 4D) and that intracellular calcium, indeed, increased (supplemental Figure 1). The PMCA4-specific inhibitor aurin–tricarboxylic acid was ineffective (supplemental Figure 2), but mouse RBCs express predominantly PMCA1 in contrast to human RBCs that have both PMCA1 and 4.42,43 Adding C16-ceramide in the same concentration as those in HNF1A−/− RBCs had no effect and did not alter that of sphingosine (supplemental Figure 3A). These data suggested that reduced PMCA activity by higher sphingosine levels may contribute to the phenotype of HNF1A−/− RBCs.
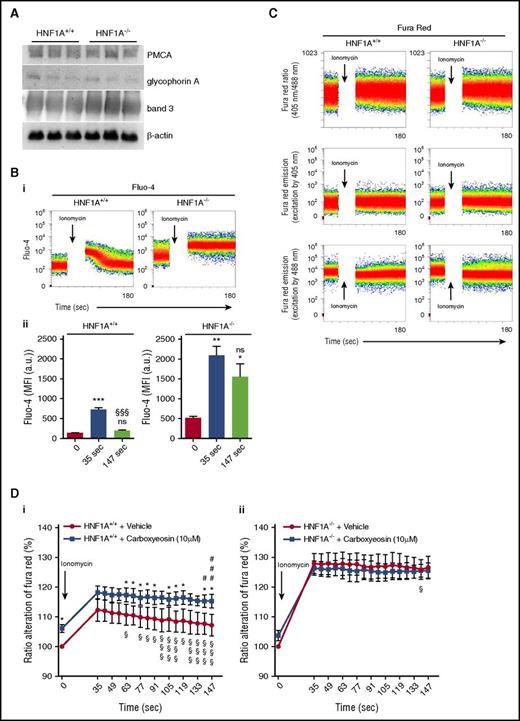
HNF1A−/− RBCs exhibit increased intracellular calcium levels due to impaired PMCA function
PMCA protein levels or those of structural proteins such as band 3 and glycophorin A did not differ between RBC ghosts from HNF1A−/− and WT RBCs (Figure 5A; supplemental Figure 4). To address PMCA activity in life RBCs, we first performed kinetic studies in Fluo-4–loaded RBCs, where we observed that calcium increased in WT RBCs after ionomycin and rapidly declined thereafter nearly to baseline levels (Figure 5B). In contrast, HNF1A−/− RBCs had an increased steady-state calcium level, which still increased after ionomycin but, strikingly, did not decline with time (Figure 5B), suggesting functional PMCA impairment.
Effects of carboxyeosin on intracellular calcium levels in HNF1A+/+and HNF1A−/−RBCs. (A) Immunoblot analysis showing PMCA, band 3, glycophorin A, and β-actin protein expression in RBC ghosts prepared from HNF1A+/+ and HNF1A−/− mice (n = 3 each). (B) Representative FACS plots of intracellular calcium kinetics measured in Fluo-4–loaded HNF1A+/+ and HNF1A−/− RBCs under basal conditions and after subsequent ionomycin treatment (i). Flow cytometric determination of intracellular Ca2+ changes over time as measured by the emission of Fluo-4–loaded HNF1A+/+ (n = 4) and HNF1A−/− RBCs (n = 4) under basal conditions (set as t = 0) and after subsequent ionomycin treatment (t = 35 seconds and t = 147 seconds) (ii). ***P < .001 for untreated HNF1A+/+ vs treated HNF1A+/+. §§§P < .001 for t = 35 seconds vs t = 147 seconds HNF1A+/+. *P < .05 and **P < .01 for untreated vs treated HNF1A−/−. (C) FACS plots of intracellular calcium kinetics measured in Fura Red–loaded HNF1A+/+ and HNF1A−/− RBCs as emission ratio (405 nm/488 nm) and fluorescence emission intensities at each of the 2 component wavelengths (FL10 excited by 405 nm and FL4 excited by 488 nm) under basal conditions and after ionomycin. (D) Flow cytometric determination of intracellular Ca2+ changes over time as measured by the ratio alteration of Fura Red–loaded HNF1A+/+ (n = 3) (i) and HNF1A−/− (n = 3) (ii) RBCs after incubation for 30 minutes (set as 100%) with the PMCA inhibitor carboxyeosin (CE) (10 μM) or vehicle (DMSO) under basal conditions (set as t = 0) and after subsequent ionomycin treatment. *P < .05 for untreated HNF1A+/+ vs treated HNF1A+/+ RBCs. §P < .05 and §§§P < .001 for ionomycin-treated RBCs (t = 35 seconds vs every later time point). #P < .05 and ###P < .001 for ionomycin- and CE-treated RBCs (t = 35 seconds vs every later time point).
Effects of carboxyeosin on intracellular calcium levels in HNF1A+/+and HNF1A−/−RBCs. (A) Immunoblot analysis showing PMCA, band 3, glycophorin A, and β-actin protein expression in RBC ghosts prepared from HNF1A+/+ and HNF1A−/− mice (n = 3 each). (B) Representative FACS plots of intracellular calcium kinetics measured in Fluo-4–loaded HNF1A+/+ and HNF1A−/− RBCs under basal conditions and after subsequent ionomycin treatment (i). Flow cytometric determination of intracellular Ca2+ changes over time as measured by the emission of Fluo-4–loaded HNF1A+/+ (n = 4) and HNF1A−/− RBCs (n = 4) under basal conditions (set as t = 0) and after subsequent ionomycin treatment (t = 35 seconds and t = 147 seconds) (ii). ***P < .001 for untreated HNF1A+/+ vs treated HNF1A+/+. §§§P < .001 for t = 35 seconds vs t = 147 seconds HNF1A+/+. *P < .05 and **P < .01 for untreated vs treated HNF1A−/−. (C) FACS plots of intracellular calcium kinetics measured in Fura Red–loaded HNF1A+/+ and HNF1A−/− RBCs as emission ratio (405 nm/488 nm) and fluorescence emission intensities at each of the 2 component wavelengths (FL10 excited by 405 nm and FL4 excited by 488 nm) under basal conditions and after ionomycin. (D) Flow cytometric determination of intracellular Ca2+ changes over time as measured by the ratio alteration of Fura Red–loaded HNF1A+/+ (n = 3) (i) and HNF1A−/− (n = 3) (ii) RBCs after incubation for 30 minutes (set as 100%) with the PMCA inhibitor carboxyeosin (CE) (10 μM) or vehicle (DMSO) under basal conditions (set as t = 0) and after subsequent ionomycin treatment. *P < .05 for untreated HNF1A+/+ vs treated HNF1A+/+ RBCs. §P < .05 and §§§P < .001 for ionomycin-treated RBCs (t = 35 seconds vs every later time point). #P < .05 and ###P < .001 for ionomycin- and CE-treated RBCs (t = 35 seconds vs every later time point).
To test whether this was due to differences in PMCA activity between the genotypes, we compared the response of WT and HNF1A−/− RBCs to the more specific PMCA inhibitor carboxyeosin under basal conditions and after treatment with ionomycin.44 The use of carboxyeosin precludes calcium measurements with Fluo-4 as it emits a fluorescent FL1 signal similar to that of Fluo-4.45 Thus, we established a calcium assay with several modifications based on Fura Red/AM and the ratiometric analysis of Violet and Blue laser excitation (Figure 5C) similar to studies in human leukocytes.24 Validity of the assay was ensured by lack of bleed-through and neglectable carboxyeosin fluorescence (supplemental Figure 5) and a calcium calibration curve in permeabilized RBCs (supplemental Figure 6). We observed that WT RBCs treated for 30 minutes with carboxyeosin featured higher calcium levels than untreated controls (Figure 5Di), suggesting that PMCA was required for steady-state calcium export. In contrast, HNF1A−/− RBCs exhibited no difference in Fura Red ratio alteration in the absence and presence of carboxyeosin (Figure 5Dii). Kinetic studies with ionomycin revealed that in WT RBCs, calcium increased in the absence and presence of carboxyeosin to the same extent and declined thereafter in the absence but not presence of carboxyeosin (Figure 5Di). This is in agreement with PMCA-dependent calcium extrusion and similar to findings in platelets.44 In contrast, HNF1A−/− RBCs exhibited no decline in Fura Red ratio alteration after ionomycin, irrespective of carboxyeosin over the entire observation time (Figure 5Dii).
Alterations in genes involved in sphingosine metabolism in HNF1A deficiency
To understand how HNF1A deficiency may affect erythroid differentiation and sphingolipid metabolism, we performed transcriptional analysis of numerous genes involved in RBC differentiation and sphingolipid regulatory pathways, respectively, in isolated erythroid precursors and liver tissue from HNF1A−/− and WT mice (supplemental Table 2; Table 3). Although no changes were observed in erythroid precursors, the expression of 4 genes critically important in sphingolipid metabolism was deregulated in HNF1A−/− livers: Ormdl1, sphingosine kinase 2 (Sphk2), neutral ceramidase, and ceramide synthase-5 (Table 3). The first 2 genes are previously unrecognized direct targets of HNF1A according to the TRANSFAC Predicted Transcription Factor Targets data set and bear the HNF1A transcriptional motif in their promotors (the inverted palindrome 5′-GTTAATNATTAAC-3′).46 Accordingly, gene expression of Ormdl1 and Sphk2 was downregulated by 34% and 42%, respectively (Table 3). ORMDL1 is an inhibitor of the serine palmitoyltransferase (SPT), the first and rate-limiting enzyme of de novo sphingomyelin biosynthesis,47 whereas Sphk2 is the predominant sphingosine kinase in the liver and metabolizes sphingosine to S1P.48 The other 2 genes, neutral ceramidase and ceramide synthase-5, were both significantly upregulated (twofold and 1.8-fold; Table 3), probably due to compensatory mechanisms as suggested for some other 415 upregulated genes in HNF1A−/− livers along with 165 downregulated ones according to an analysis of 25 177 gene sequences.49
Gene expression in livers of HNF1A+/+ and HNF1A−/− mice
| Gene . | HNF1A+/+ ΔCT, n = 3 . | HNF1A−/− ΔCT, n = 3 . | x-fold . |
|---|---|---|---|
| Sphk2 | 4.76 ± 0.23 | 5.61 ± 0.21 | 0.58 ± 0.09* |
| Sphk1 | 9.95 ± 0.67 | 8.87 ± 0.28 | 2.31 ± 0.41 |
| Ormdl1 | 4.50 ± 0.15 | 5.16 ± 0.18 | 0.66 ± 0.08* |
| Asah2 | 6.62 ± 0.15 | 5.72 ± 0.23 | 1.99 ± 0.29* |
| Cers5 | 7.51 ± 0.14 | 6.72 ± 0.19 | 1.81 ± 0.24* |
| Sgpp2 | 4.02 ± 0.31 | 4.59 ± 0.14 | 0.69 ± 0.07 |
| Sgpp1 | 18.06 ± 0.43 | 17.0 ± 0.40 | 2.59 ± 0.84 |
| Spns2 | 8.79 ± 0.26 | 8.29 ± 0.23 | 1.50 ± 0.25 |
| Asah1 | 3.75 ± 0.36 | 3.43 ± 0.13 | 1.28 ± 0.12 |
| Scarb1 | 2.24 ± 0.34 | 2.36 ± 0.27 | 0.99 ± 0.16 |
| Cers2 | 1.43 ± 0.15 | 2.36 ± 0.15 | 0.54 ± 0.05 |
| Smpd2 | 5.40 ± 0.15 | 5.67 ± 0.14 | 0.85 ± 0.08 |
| Smpd1 | 4.13 ± 0.12 | 4.19 ± 0.12 | 0.98 ± 0.08 |
| Sgms1 | 5.01 ± 0.17 | 4.87 ± 0.07 | 1.10 ± 0.05 |
| Sgms2 | 7.64 ± 0.21 | 8.03 ± 0.18 | 0.79 ± 0.10 |
| Spt | 4.82 ± 0.29 | 4.88 ± 0.12 | 0.98 ± 0.09 |
| Cers4 | 6.17 ± 0.28 | 6.67 ± 0.06 | 0.71 ± 0.03 |
| Nogo-A | 12.40 ± 1.09 | 14.25 ± 0.10 | 0.28 ± 0.02 |
| Nogo-B1 | 5.63 ± 0.36 | 5.21 ± 0.17 | 1.38 ± 0.15 |
| Gene . | HNF1A+/+ ΔCT, n = 3 . | HNF1A−/− ΔCT, n = 3 . | x-fold . |
|---|---|---|---|
| Sphk2 | 4.76 ± 0.23 | 5.61 ± 0.21 | 0.58 ± 0.09* |
| Sphk1 | 9.95 ± 0.67 | 8.87 ± 0.28 | 2.31 ± 0.41 |
| Ormdl1 | 4.50 ± 0.15 | 5.16 ± 0.18 | 0.66 ± 0.08* |
| Asah2 | 6.62 ± 0.15 | 5.72 ± 0.23 | 1.99 ± 0.29* |
| Cers5 | 7.51 ± 0.14 | 6.72 ± 0.19 | 1.81 ± 0.24* |
| Sgpp2 | 4.02 ± 0.31 | 4.59 ± 0.14 | 0.69 ± 0.07 |
| Sgpp1 | 18.06 ± 0.43 | 17.0 ± 0.40 | 2.59 ± 0.84 |
| Spns2 | 8.79 ± 0.26 | 8.29 ± 0.23 | 1.50 ± 0.25 |
| Asah1 | 3.75 ± 0.36 | 3.43 ± 0.13 | 1.28 ± 0.12 |
| Scarb1 | 2.24 ± 0.34 | 2.36 ± 0.27 | 0.99 ± 0.16 |
| Cers2 | 1.43 ± 0.15 | 2.36 ± 0.15 | 0.54 ± 0.05 |
| Smpd2 | 5.40 ± 0.15 | 5.67 ± 0.14 | 0.85 ± 0.08 |
| Smpd1 | 4.13 ± 0.12 | 4.19 ± 0.12 | 0.98 ± 0.08 |
| Sgms1 | 5.01 ± 0.17 | 4.87 ± 0.07 | 1.10 ± 0.05 |
| Sgms2 | 7.64 ± 0.21 | 8.03 ± 0.18 | 0.79 ± 0.10 |
| Spt | 4.82 ± 0.29 | 4.88 ± 0.12 | 0.98 ± 0.09 |
| Cers4 | 6.17 ± 0.28 | 6.67 ± 0.06 | 0.71 ± 0.03 |
| Nogo-A | 12.40 ± 1.09 | 14.25 ± 0.10 | 0.28 ± 0.02 |
| Nogo-B1 | 5.63 ± 0.36 | 5.21 ± 0.17 | 1.38 ± 0.15 |
Gene expression was measured by real-time PCR. Data are presented as ∆CT (mean of n = 3) and x-fold (ratio of the 2 means) and are normalized to Rn18s.
CT, cycle threshold.
P < .05 for HNF1A+/+ vs HNF1A−/−.
Bone marrow transplantation rescues HNF1A−/− mice from anemia
To address the question of whether the anemia in HNF1A−/− mice was caused by changes in the whole-body sphingolipidome or by a RBC-autonomous effect, we analyzed RBCs in cohorts of bone marrow transplanted mice that we previously used to study B-cell lymphopoiesis in HNF1A−/− mice.18 Interestingly, there were no differences in any RBC parameters between WT→WT and KO→WT bone marrow chimera (Table 4), suggesting that bone marrow transplantation had rescued the phenotype. Furthermore, we measured RBC sphingolipid concentrations in bone marrow transplanted mice and, again, observed no differences between WT→WT and KO→WT bone marrow chimera (Table 4). In fact, all sphingolipids that were elevated in regular HNF1A−/− RBCs were back to normal in RBCs from KO→WT chimera (Table 4). Unfortunately, none of the WT→KO chimera survived due to procedural stress in an already compromised HNF1A−/− strain. We circumvented this by testing the osmotic fragility of WT RBCs exposed to HNF1A−/− plasma. Remarkably, HNF1A−/− plasma deteriorated their osmotic fragility (supplemental Figure 7). WT plasma did not improve the osmotic fragility of HNF1A−/− RBCs (supplemental Figure 7), although KO→WT bone marrow transplantation did rescue the HNF1A−/− phenotype, suggesting longer time necessary or additional mechanisms in vivo. These data clearly argue in favor of a non–cell-autonomous RBC defect secondary to the sphingolipidome changes caused by HNF1A deficiency.
Characteristics of peripheral blood and RBC sphingolipid concentration 6 weeks after bone marrow transplantation
| Genotype . | HNF1A+/+ → HNF1A+/+, n = 6 . | HNF1A−/− → HNF1A+/+, n = 5 . |
|---|---|---|
| Blood parameters | ||
| HCT, % | 48.2 ± 3.2 | 45.6 ± 2.9 |
| HGB, g/dL | 16.7 ± 1.1 | 15.5 ± 1.4 |
| RBC, (×106)/µL blood | 11.2 ± 0.8 | 10.4 ± 0.3 |
| MCV, µm3 | 50.0 ± 1.4 | 52.4 ± 0.7 |
| MCH, pg | 15.1 ± 0.3 | 14.9 ± 0.6 |
| MCHC, g/dL | 30.2 ± 0.3 | 28.5 ± 1.3 |
| RDW, % | 14.2 ± 1.1 | 13.6 ± 0.2 |
| Sphingolipids, nM | ||
| Sphingosine | 4 321 ± 436 | 4 614 ± 236 |
| C16-ceramide | 539 ± 108 | 510 ± 77 |
| Sphingomyelin | 241 000 ± 12 959 | 230 560 ± 9 788 |
| Genotype . | HNF1A+/+ → HNF1A+/+, n = 6 . | HNF1A−/− → HNF1A+/+, n = 5 . |
|---|---|---|
| Blood parameters | ||
| HCT, % | 48.2 ± 3.2 | 45.6 ± 2.9 |
| HGB, g/dL | 16.7 ± 1.1 | 15.5 ± 1.4 |
| RBC, (×106)/µL blood | 11.2 ± 0.8 | 10.4 ± 0.3 |
| MCV, µm3 | 50.0 ± 1.4 | 52.4 ± 0.7 |
| MCH, pg | 15.1 ± 0.3 | 14.9 ± 0.6 |
| MCHC, g/dL | 30.2 ± 0.3 | 28.5 ± 1.3 |
| RDW, % | 14.2 ± 1.1 | 13.6 ± 0.2 |
| Sphingolipids, nM | ||
| Sphingosine | 4 321 ± 436 | 4 614 ± 236 |
| C16-ceramide | 539 ± 108 | 510 ± 77 |
| Sphingomyelin | 241 000 ± 12 959 | 230 560 ± 9 788 |
Data are presented as mean ± SEM and were analyzed for significant differences with a Student t test.
Discussion
We provide for the first time evidence that HNF1A is required for RBC homeostasis in a nonautonomous cell manner. HNF1A−/− mice displayed hypochromic microcytic anemia characterized by abnormally shaped, structurally and biochemically altered as well as osmotically fragile RBCs. Several HNF1A−/− tissues (RBCs, liver, and plasma) revealed complex changes of the sphingolipidome in a tissue-specific manner with high sphingosine as 1 common denominator. The evidence clearly supports a nonautonomous HNF1A−/− RBC defect caused secondarily by sphingolipid disturbances: (i) all phenotypic changes of HNF1A−/− RBCs (osmotic fragility, PS exposure, and calcium increase) could be simulated by exposing WT RBCs to sphingosine; (ii) HNF1A−/− bone marrow transplantation into WT hosts rescued the anemia; (iii) potent extramedullary erythropoiesis took place in HNF1A−/− spleens, suggesting that shortened RBC life span (although not directly shown) rather than defects in erythropoiesis is the cause of anemia; (iv) expression of erythroid differentiation genes was not altered in HNF1A−/− erythroid precursors. Other causes of anemia such as iron or erythropoietin deficiency were excluded, which is important as HNF1A−/− mice have renal dysfunction similar to human renal Fanconi syndrome (although without increase in plasma creatinine).3 HNF1A−/− mice also display multiple liver functional defects3,49 but we observed no intrahepatic or intraperitoneal bleeding or adenomas, excluding intrahepatic hemorrhagy as cause for anemia (described in a MODY3 patient with liver adenomatosis22 ).
PS exposure and calcium increase are associated with various biological processes and their subordination to a specific process termed eryptosis has been controversial. We have observed PS exposure, increased calcium, diminished cell size, and higher ceramide concentrations in HNF1A−/− RBCs but an identical response to hyperosmolaric stress and protection by aSMase inhibitors, respectively, as in WT RBCs. KO RBCs also featured enhanced osmotic fragility and not an attenuated one as in eryptosis.31 Thus, our observations do not concur with eryptosis but indicate other alterations in the mechanical, biochemical, and functional properties of HNF1A−/− RBCs.
Along with the elevation of C16-ceramide, we have observed an accumulation of sphingosine, a ceramide precursor of 15-fold higher abundance. Although sphingosine has been suggested to induce eryptosis in human RBCs,36 this occurred after days and without increases in cation conductance36 that does not concur with the activation of calcium-permeable cation channels in eryptosis. We thus hypothesized that high sphingosine may raise calcium through PMCA inhibition based on studies showing inhibition of PMCA by sphingosine in purified enzyme preparations.38,41 Indeed, HNF1A−/− RBCs featured lower PMCA activity: (i) studies using Fluo-4 as well as Fura Red/AM showed a decline of calcium with time after the initial ionomycin-induced raise only in WT but not HNF1A−/− RBCs, and (ii) carboxyeosin prevented calcium extrusion after ionomycin in WT RBCs but had no additional effect in HNF1A−/− RBCs. Ceramides such as the unnatural C2-ceramide and to a lesser extent C8-ceramide have been reported to enhance PMCA activity in purified enzyme preparations dependent on chain length and at equimolar concentrations as sphingosine.41 We did not see any effects of ceramide-16 (the ceramide found elevated in HNF1A−/− RBCs) when added to WT RBCs at the same concentration as that present in HNF1A−/− RBCs (supplemental Figure 3).
Increased calcium activates the calcium-sensitive K+ channel (the Gardos channel) increasing K+ efflux and causing RBC dehydration that normally reduces osmotic fragility. However, under certain conditions such as elevated intracellular calcium, there is decoupling between cell hydration state and osmotic fragility, with elevated calcium causing an increase in osmotic fragility with only minimal contribution from cellular hydration, which has been interpreted as a specific calcium-induced lytic vulnerability of the membrane.40 In our case, the lower MCV of HNF1A−/− RBCs may result from Gardos channel activation, whereas the increased osmotic fragility would reflect the calcium-specific lytic effect. Accordingly, vanadate as a nonspecific but potent PMCA inhibitor increased calcium and osmotic fragility in WT RBCs in agreement with its effect in calcium-preloaded human RBCs.40 Interestingly, vanadate has been shown to induce echinocytosis in human RBCs through calcium elevation,50 a phenotype resembling that of HNF1A−/− RBCs. Although activation of the Gardos channel would have been expected to decrease adenosine triphosphate (ATP) in HNF1A−/− RBCs due to the Na,K-ATPase trying to restore potassium equilibrium, ATP levels remained unchanged (supplemental Figure 8) suggesting that a compensatory mechanism replenishes ATP. One possibility may be through stimulation of glycolysis by the high S1P concentrations in HNF1A−/− RBCs as shown for RBCs in sickle cell disease (SCD).51
In humans, several forms of hereditary hemolytic anemia such as SCD, β-thalassemia, or phosphofructokinase deficiency are associated with increases in intracellular calcium that cannot be compensated by increases in PMCA activity.52 Intriguingly, in human SCD and mouse SCD models, not only S1P but also sphingosine accumulates in RBCs and, although S1P was shown to contribute to sickling, the role of sphingosine was not addressed.53 Whereas increased sphingosine concentrations are a plausible cause of reduced PMCA activity in HNF1A−/− RBCs, sphingosine may certainly have other effects than promoting osmotic fragility independently of PMCA, such as its ability to increase cell membrane permeability through rigidification of gel domains leading to structural defects during the lateral phase separation of “more” and “less” rigid membrane domains.54 So far, there are no data available on RBC characteristics of mice deficient for any PMCA isoforms.55 PMCA has not been reported as a HNF1A target gene, and we have found no reduction in protein expression.
The causes for the altered sphingolipid content in RBCs, livers, and plasma of HNF1A−/− mice are certainly complex. We have identified 4 genes related to sphingolipid metabolism that are transcriptionally deregulated in HNF1A−/− livers. Two of them, Ormdl1 and Sphk2, are previously unrecognized direct HNF1A targets according to the TRANSFAC Predicted Transcription Factor Targets data set.46 Accordingly, we found their gene expression to be downregulated. ORMDL1 is an inhibitor of the SPT, the first and rate-limiting enzyme of de novo sphingomyelin biosynthesis,47 whereas Sphk2 metabolizes sphingosine to S1P.48 Three different genes encode ORMDL proteins (ORMDL1-3)56 and the yeast orthologous Orm1 and Orm2 are known to inhibit SPT.47 Deletion of orosomucoids or phosphomimicking mutations causes hyperactivation of SPT and increases production of sphingolipids.47 In mammalian cells, the ORMDL proteins have similar negative regulatory functions on SPT.57 Silencing all 3 results in increased ceramide levels47 through increased sphingolipid synthesis, whereas treatment with C6-ceramide represses SPT through ORMDLs.58 Thus, ORMDLs have a direct role in translating elevated cellular sphingolipid levels into inhibition of SPT.57 However, the consequences of ORMDL downregulation on steady-state sphingolipid levels is complex: strong elevation of dihydrosphingosine-1-phosphate (dhS1P) has been described and interpreted as an indication that activated SPT generates dihydrosphingosine (dhSph) that is then quickly phosphorylated by sphingosine kinases shunting it away prior to use as ceramide precursor. In addition, S1P was also shown to increase due to high ceramidase activity presumably as an attempt to prevent high ceramide levels.57 In contrast to ORMDL depletion, sphingosine kinase depletion leads to increased ceramide, which has been interpreted as a mechanism of control over ceramide levels by generation of S1P (the only substrate suitable for irreversible degradation of the sphingolipid backbone by the S1P lyase).57 Interestingly, the other gene we have observed as downregulated in HNF1A deficiency was, indeed, Sphk2. Assuming this also takes place in HNF1A−/− mice, the joint downregulation of Ormdl and Sphk2 would result in increased dhSph production but ineffective shunting to dhS1P, which leads to increased ceramide synthesis. Furthermore, sphingosine production would also be enhanced as ceramidases respond best to a combination of ORMDL downregulation and C6-ceramide stimulation.57 The result would be both higher ceramide and sphingosine, a constellation that we, indeed, have observed in HNF1A deficiency. The 2 other genes we observed to be altered, neutral ceramidase and ceramide synthase-5, may be upregulated due to compensatory mechanisms, for example, through enhanced ceramide turnover. Finally, HNF1A−/− mice completely lack apolipoprotein M,49 a transcriptional HNF1A target and quintessential S1P-binding protein in plasma and high-density lipoproteins (HDLs).59 Accordingly, S1P concentrations in plasma and HDL are reduced25 possibly resulting from inefficient S1P tissue unloading with consecutive accumulation of sphingosine and ceramide.
Human heterozygous HNF1A mutations lead to early-onset diabetes mellitus type MODY3.4,5 Some human mutations have been associated with increased production of bile acids60 and higher plasma HDL cholesterol levels,61 whereas loss-of-function mutations have been linked to hepatic tumorigenesis.62-64 Genome-wide association studies have implicated HNF1A in the development of pancreatic cancer,65 coronary artery disease,66 and low-density lipoprotein hypercholesterolemia.67 So far, no systematic investigations have addressed RBC abnormalities in human HNF1A mutations, although there has been a report of microcytic anemia of unknown etiology in a woman with MODY3,21 and a case report of a MODY3 patient with liver adenomatosis and low hemoglobin caused by intrahepatic hemorrhage.22 Our data will hopefully encourage clinical research in MODY3 patients to explore the as-yet unrecognized hematological consequences of human HNF1A deficiency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Abou Hamed for excellent technical help.
This work was supported by the Deutsche Forschungsgemeinschaft (LE940/4-2, Graduiertenkolleg 2098, projects 8, 9, 10) (B.L.).
Authorship
Contribution: K.v.W.L. and B.L. conceived and designed the research studies, analyzed and interpreted data, wrote the manuscript, and made the figures and tables; K.v.W.L., S.W., S.P., P.K., H.A.B., and M.H.G. performed experiments and acquired and analyzed data; and B.L., K.v.W.L., and G.H. interpreted data and wrote and critically edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bodo Levkau, Institute for Pathophysiology, West German Heart and Vascular Center, University Hospital Essen, Hufelandstr 55, 45122 Essen, Germany; e-mail: bodo.levkau@uni-due.de.

![Figure 1. Anemia, reticulocytosis, and analysis of hematopoietic stem cells, MEPs, and erythroid cell populations in the bone marrow of HNF1A−/− mice. (A) Flow cytometric reticulocyte quantification using thiazole orange in HNF1A+/+ and HNF1A−/− mice (n = 7 each). Representative images of Ter119+Thiazole orange+ reticulocytes (i), quantification of reticulocytes in percentage (ii) and total numbers per microliter of whole blood (iii). (B) Calculation of mature RBC counts per microliter of blood as performed by subtraction of reticulocyte counts from total RBC counts. (C) Representative images and gating strategy for LSK (Lin−Sca-1+c-Kit+IL-7R−) cells and MEPs (Lineage−IL-7R−Sca-1−c-Kit+CD34−CD16/32−/low [FcγRII/III]) in bone marrow from HNF1A−/− and HNF1A+/+ mice (n = 6 each) as analyzed by flow cytometry (i). LSK and MEP cell populations were determined according to Kondo et al,26 Pronk et al,27 and Murphy et al28 after eliminating lineage-positive cells (Lin−: CD11b, Gr-1, CD49b, CD11c, CD3ε, CD4, CD8, B220, Ter119). Percentage (ii) and total cell numbers per femur length (iii) of LSKs and MEPs as provided. Cell counts per femur were divided by femur length to normalize for the shorter HNF1A−/− femura. (D) Representative fluorescence-activated cell sorter (FACS) plots and gating strategy of erythroid cell populations by combination of Ter119 and CD71 expression and determination of cell size in regions I to VI, respectively, as described.29,30 Erythroid populations were distinguished in early proerythroblasts (Pro; Ter119medFSChighCD71high), basophilic erythroblasts (Baso; Ter119highFSChighCD71high), polychromatic erythroblasts (Poly; Ter119highFSCmed/highCD71med/high), orthochromatic erythroblasts (Ortho; Ter119highFSCmedCD71med), reticulocytes (Retic; Ter119highFSClowCD71low), and mature RBCs (Ter119highFSClowCD71−) (i). Total cell numbers of proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts (ii), orthochromatic erythroblasts, reticulocytes, and mature RBCs (iii) per femur normalized for femur length are provided as indicated. All data are presented as mean ± SEM. *P < .05, **P < .01, and ***P < .001 for HNF1A+/+ vs HNF1A−/− (Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/25/10.1182_blood-2017-03-774356/4/m_blood774356f1.jpeg?Expires=1769126737&Signature=AhAaCvT2uNtfBsk-wd4I64ZoDT3EmqWDuO1jhX~LDk6Q1w6e5Yav9PCEs3Qv6~dH0zOWrbhGbDQOJg7kTyAm8~u4R2G~4mV4x9Ud2Tf5cnyWRab7Vb1gOKNP~cpE5j5NlIYv6t3KHEKF8zXWhmW2ZGmcW7gU9VK-CfgxLDNu7SUA5~OmbgzXRjrkUT~dLFh4N2HQ3DsVn5T~Bi45e0koG1MP2ig5d-3Ts6OheqtqpgTZFl~UlbvZpEr8oz~fxDCVlvAQjWSZKRn~z5iEjZwB-vYsnSYn5jJ8dF9A8F-A7J4yXeCwmNbXC9~X18JPlf1CZ0C7imdGlAtjINkCAFCneA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal