In a comprehensive study in this issue of Blood, Carden and colleagues describe the importance of the tonicity of IV fluids used in the treatment of patients with sickle cell disease (SCD) during vaso-occlusive crises (VOCs). Hypertonic fluids decreased sickle red blood cell (sRBC) deformability, increased occlusion, and increased sRBC adhesion in microfluidic human microvasculature models. Hypotonic fluids decreased sRBC adhesion but prolonged sRBC transit time. Fluids with intermediate tonicities resulted in optimal changes that reduced the risk of vaso-occlusion.1
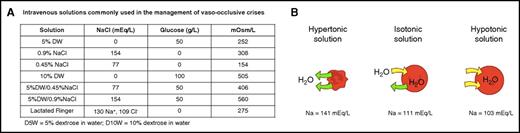
Intravenous solutions and the effect of tonicity of extracellular fluids on RBC. (A) IV fluids that are usually used to treat hydration in patients with SCD. (B) RBCs suspended in hypertonic solution lose water, shrink, and are transformed to dehydrated xerocytes. Suspended in hypotonic solution, RBCs gain water, swell, and are transformed to spherocytes. Panel B adapted from OpenStax11 (source: Mariana Ruiz Villareal; licensed under a Creative Commons Attribution License 4.0). Download for free at http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.118.
Intravenous solutions and the effect of tonicity of extracellular fluids on RBC. (A) IV fluids that are usually used to treat hydration in patients with SCD. (B) RBCs suspended in hypertonic solution lose water, shrink, and are transformed to dehydrated xerocytes. Suspended in hypotonic solution, RBCs gain water, swell, and are transformed to spherocytes. Panel B adapted from OpenStax11 (source: Mariana Ruiz Villareal; licensed under a Creative Commons Attribution License 4.0). Download for free at http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.118.
Osmosis is the movement of a neutral solvent across a semipermeable (red blood cell [RBC]) membrane from a less concentrated solution into a more concentrated one. This movement equalizes the concentration of solutes on each side of the membrane. Tonicity, on the other hand, is the ability of a solution to make water move into or out of a cell by osmosis. It depends on the concentration of all solutes in the solution (ie, its osmolarity). A solution with low osmolarity is hypotonic and vice versa. Solutions having the same osmolarity are isotonic.2,3
Water in pools and rivers is hypotonic compared with sea water, but water from the Dead Sea is hypertonic. Swimming in the ocean or sea is relatively easier than swimming in a pool or river due to the buoyancy of seawater, which contains ∼3.5% salt. Floating, rather than swimming, is the rule in the Dead Sea due to its hypertonicity with ∼34.2% salt. Metaphorically, the RBC maintains its normal shape, shrinks, or balloons depending on the tonicity of the extracellular solution in which it is suspended.
It is generally believed that aggressive treatment of the VOC at its onset would shorten its duration with fewer complications.4 The rational approach to abort a VOC is to treat it as early as possible, when tissue ischemia and inflammation are in their early stages. The standard of care of anti-VOC therapy to abort a crisis includes a trial of hydration, anti-inflammatories, analgesics, and adjuvants.4 Hydration often requires the administration of IV fluids. Fluids that are usually used for hydration are shown in figure panel A. The choice of a certain fluid varies greatly among institutions and providers. The recent guidelines of the National Heart, Lung, and Blood Institute to treat the complications of SCD did not adequately address this issue.5,6 In addition, a recent Cochrane review of fluid replacement therapy for acute episodes of pain in people with SCD found no randomized controlled trials that have assessed the safety and efficacy of different routes, types, or quantities of fluid.7
In this issue of Blood, Carden et al describe elegant studies to determine the tonicity of the desirable IV fluid to hydrate patients with VOC. The scheme is illustrated in figure panel B. sRBC exposed to admixtures with the highest sodium level of 141 mEq/L (hypertonic solution) impacted sRBC biomechanics due to dehydration (xerocytosis), with increased mean corpuscular hemoglobin concentration (MCHC) and decreased mean corpuscular volume associated with increased cytoplasmic viscosity and increased membrane rigidity leading to decreased deformability and increased tendency for occlusion under normoxic and hypoxic conditions in the microfluidic human microvascular models used by the authors. This is similar to the xerocytosis described in hemoglobin SCD.8 On the other hand, the exposure of sRBC admixtures to hypotonic solution with a sodium level of 103 mEq/L caused water gain with a decreased surface area-to-volume ratio, transforming sRBCs to swollen spherocytes with significantly decreased deformability and increased risk of occlusion in the normoxic microfluidic models used due to the fact that overswollen spherocytes prolonged the transit times of sRBCs in capillary-sized microchannels. Admixtures with sodium concentrations of 111 to 122 mEq/L appeared to optimize changes in sRBC deformability, in part due to ideal changes in the MCHC, the most sensitive predictor of hemoglobin S polymerization and microvascular occlusion, and in part due to the optimization of the surface area-to-volume ratio necessary for sRBCs to traverse capillary microchannels.
In addition to the salutary effects of isotonic IV fluids to treat VOCs by preventing or minimizing vaso-occlusion, the authors proceeded to show the effects of the tonicity of IV fluids on the adhesion of sRBCs to the vascular endothelium and laminin. Admixtures of sRBCs with a sodium concentration of 141 mEq/L demonstrated increased adhesion to both human endothelium and laminin. On the other hand, admixtures of sRBCs with a sodium concentration of 103 mEq/L showed decreased adhesion to the endothelium and subendothelium matrix, probably because the hydrated swollen spherocytes may alter cell membrane contact points with the endothelium and subendothelium.9 This effect, however, seems to be offset by the prolongation of the transit time of swollen spherocytes in capillary-sized microchannels.
This study presents a seminal translational approach to determine the appropriate IV fluids to be used in the management of patients with SCD during VOCs. The authors are aware that their study did not specify the type of sRBCs, such as reticulocytes, dense cells, etc., and hope to overcome these limitations in future projects. They are also aware that plasma components affect the rheology of sRBCs beyond their effect on tonicity.10 The authors did specify the IV route of fluid administration and the tonicity of the fluids used. They did not, however, address the quantity to be used and followed during hospitalization. Patients with sickle cell anemia are known to have an inability to concentrate urine. Urinary osmolality of 400 to 450 mOsm/kg is often seen in adult patients with sickle cell anemia after water deprivation conditions. Perhaps this should be considered in choosing the tonicity of the fluids to be used for hydration.
This well-designed study indicates the need for clinical randomized trials to determine the safety and efficacy of different routes, types, and quantities of fluids administered to patients with different types of SCD during VOCs.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal