In this issue of Blood, Carey et al demonstrate that tumor-associated macrophages represent the quantitatively predominant cell type that expresses programmed death 1 ligand (PD-L1) in classical Hodgkin lymphoma (CHL) contributing to the formation of a favorable microenvironment for malignant Hodgkin Reed-Sternberg (HRS) cells.1
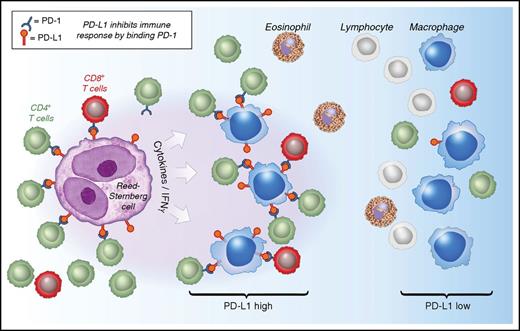
Model of interactions between PD-1 and PD-L1 in CHL. See Figure 6 in the article by Carey et al that begins on page 2420.
Model of interactions between PD-1 and PD-L1 in CHL. See Figure 6 in the article by Carey et al that begins on page 2420.
CHL is characterized by the presence of malignant HRS cells, which, in contrast to almost all other cancers, represent only a minor portion (∼1%) of the tumor mass. The vast majority of the cellular infiltrate (∼99%) is composed of a spectrum of different immune cells (eg, fibroblasts, lymphocytes, macrophages) forming a protumor microenvironment (TME).2 Recent studies have demonstrated that the specific composition of the TME can be associated with treatment outcomes, and specifically, the number of tumor-associated macrophages has been shown in multiple studies to predict shorter survival after standard-of-care chemotherapy.3 The biological and clinical importance of the TME is highlighted by novel therapeutic approaches designed to target the various constituent elements of and the cellular crosstalk within the TME. Most prominently, the excitement about microenvironment targeting in CHL was fueled by impressive objective response rates (ORRs) to checkpoint inhibitor therapy targeting the programmed death 1 (PD-1) receptor axis. In contrast to other cancers, CHL represents a positive outlier, with ORRs exceeding 60% in patients with relapsed disease treated with anti–PD-1 therapeutic antibodies nivolumab and pembrolizumab.4 However, the exact mechanism of action of checkpoint inhibitors and the preferentially favorable responses in CHL remain only partially understood.
Carey et al offer important insight into the microenvironment biology of CHL, with potential implications for checkpoint inhibitor therapy. Using an elegant approach of chromogenic immunohistochemistry, the authors describe the spatial relationship of multiple cellular components, including HRS cells, macrophages, and T-cell subsets, in 20 cases of CHL. Importantly, they found that the majority of tissue-associated PD-L1, a cognate ligand to PD-1, is confined to tumor-associated macrophages and exceeds the PD-L1 expression of HRS cells. Moreover, their novel multicolor approach, paired with systematic image analysis to describe topography of cell types and intercellular distance, allowed for the description of regionally localized PD-L1+ macrophages that were enriched for contacts with PD-1+ T cells in the TME (see figure). This finding is consistent with the prominent participation of macrophages in formation of an immune-protective niche for the malignant HRS cells. In such a model, both PD-L1–expressing macrophages and malignant HRS cells are selected for during pathogenesis and cooperate to create an ecosystem characterized by a blunted immune response. This model is further supported by various publications that describe somatically acquired chromosome 9p gains and amplifications in HRS cells, harboring the CD274 (encoding PD-L1) and PDCD1LG2 (encoding PD-L2) loci, that are correlated with high expression of PD-L1 and PD-L2.5 Checkpoint inhibitors, such as nivolumab and pembrolizumab, are believed to interfere with crosstalk between PD-L1 and the PD-1 receptor and reengage PD-1+ T cells to participate in an active, antitumor immune response. However, the study by Carey et al also highlights a number of open questions in the field. First, given the low abundance of PD-1+ T cells in CHL tissues, it remains unclear which cells are the targets of therapeutic anti–PD-1 antibodies. Second, the frequently attributed exhaustion phenotype of PD-1+ T cells is only incompletely described, because PD-1 expression is also increased upon T-cell receptor engagement and activation. Additional phenotypic markers, such as TIM3 or LAG3, might help in further defining the functional state of PD-1+ T cells.6,7 Third, in their topographical study of cell types, Carey et al found macrophages enriched for contacts with rare PD-1+ T cells. However, such an enrichment pattern with PD-1+ T cells was not observed for the malignant HRS cells. This leaves the question of which functional CD4+ T-cell subtypes are predominant in the direct vicinity of HRS cells largely unanswered.8
Ecosystems are about symbiosis. The study by Carey et al captures a critical element of cellular symbiosis in cancer spatial relationships. Since 1985, tumor-associated macrophages have repeatedly emerged as the focal point of interest in the context of tumor evolution and outcome prediction in CHL.9 Despite a number of open questions in the field, the findings by Carey et al support the hypothesis that macrophages in the TME play an important role in the mechanism of action of checkpoint inhibitor therapy and suggest additional studies investigating macrophages as a potential predictive biomarker in the era of modern immunotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal