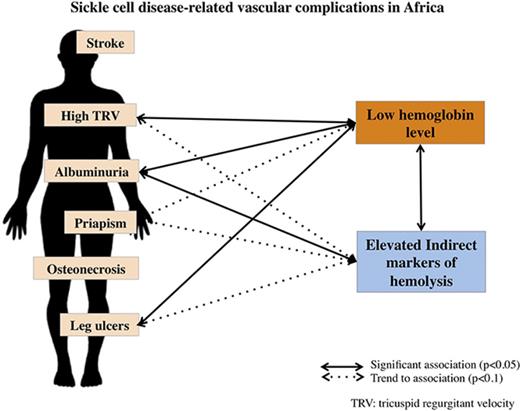
Key Points
Severe baseline anemia is associated with leg ulcer, microalbuminuria, and echographic pulmonary hypertension in African SCD patients.
These vascular complications of SCD are not independently associated with indirect markers of increased hemolysis.
Abstract
The hyperhemolysis paradigm that describes overlapping “hyperhemolytic-endothelial dysfunction” and “high hemoglobin-hyperviscous” subphenotypes of sickle cell disease (SCD) patients is based on North American studies. We performed a transversal study nested in the CADRE cohort to analyze the association between steady-state hemolysis and vascular complications of SCD among sub-Saharan African patients. In Mali, Cameroon, and Ivory Coast, 2407 SCD patients (1751 SS or sickle β-zero-thalassemia [Sβ0], 495 SC, and 161 sickle β+-thalassemia [Sβ+]), aged 3 years old and over, were included at steady state. Relative hemolytic intensity was estimated from a composite index derived from principal component analysis, which included bilirubin levels or clinical icterus, and lactate dehydrogenase levels. We assessed vascular complications (elevated tricuspid regurgitant jet velocity [TRV], microalbuminuria, leg ulcers, priapism, stroke, and osteonecrosis) by clinical examination, laboratory tests, and echocardiography. After adjustment for age, sex, country, and SCD phenotype, a low hemoglobin level was significantly associated with TRV and microalbuminuria in the whole population and with leg ulcers in SS-Sβ0 adults. A high hemolysis index was associated with microalbuminuria in the whole population and with elevated TRV, microalbuminuria, and leg ulcers in SS-Sβ0 adults, but these associations were no longer significant after adjustment for hemoglobin level. In conclusion, severe anemia at steady state in SCD patients living in West and Central Africa is associated with elevated TRV, microalbuminuria, and leg ulcers, but these vascular complications are not independently associated with indirect markers of increased hemolysis. Other mechanisms leading to anemia, including malnutrition and infectious diseases, may also play a role in the development of SCD vasculopathy.
Introduction
Sickle cell disease (SCD) is one of the most common monogenic diseases worldwide, affecting an estimated 25 million people, including 15 million in sub-Saharan Africa.1 It is due to a single-nucleotide substitution (HBB: c.20A>T (p.Glu7Val)), affecting the sixth amino acid of the β-globin chain, leading to abnormal hemoglobin S (HbS).2 The most frequent phenotype, also known as sickle cell anemia, results from the homozygous S mutation (SS), but SCD can also result from compound heterozygosity associating HbS and HbC (SC genotype) or HbS and β-thalassemia (Sβ-thalassemia genotype). The disease is characterized by hemoglobin polymerization, which induces erythrocyte deformation in various conditions of deoxygenation, and is responsible for chronic hemolytic anemia and acute microvascular vaso-occlusions and infarctions. Moreover, systemic endothelial dysfunction develops3,4 by mechanisms that are only partially elucidated, apart from a decrease in nitric oxide bioavailability due to hemolysis, reactive oxygen species production, and intravascular inflammation.5-9 The individual baseline rate of hemolysis is stable over time.10 Two overlapping subphenotypes of SCD have been proposed11 : the “hyperviscosity” subphenotype with higher hemoglobin levels and a higher prevalence of vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis, and the “hyperhemolysis-endothelial dysfunction” subphenotype, in which patients have a pattern of lower hemoglobin levels and higher levels of hemolysis markers, such as reticulocyte count, serum lactate dehydrogenase (LDH), and bilirubin, with a higher prevalence of pulmonary hypertension, stroke, leg ulcers, and priapism. Although logical, this paradigm is still debated.12,13 Indeed, it has several limitations. First, it is based on indirect inferences about the rate of hemolysis, because the gold-standard, direct measurements of red blood cell (RBC) lifespan was never used in the studies that advanced this paradigm. Instead, several common surrogate laboratory markers were used, alone or in combination, but most do not directly correlate with measured RBC lifespan in SCD, aside from reticulocyte counts.13,14 Second, associations between an increase of these surrogate markers and clinical complications have varied widely in previous studies. For example, Nouraie et al did not observe an association between increased hemolysis and priapism or leg ulcers,15 whereas Minniti et al observed an association between pulmonary hypertension and acute chest syndrome16 that is a manifestation of the high hemoglobin-hyperviscosity subphenotype in the proposed paradigm. Third, all vascular complications have rarely been studied together. Moreover, glomerular disease, 1 of the major complications of SCD, is not included in this classification, although previous studies have demonstrated an association between albuminuria and increased hemolysis.17 Finally, this hypothesis is based on data gathered from the American SCD population and needs to be assessed in other contexts to gain general significance, especially in sub-Saharan Africa. Indeed, SCD is a significant health problem in sub-Saharan Africa, with >230 000 estimated affected births per year compared with 2600 in North America and 1300 in Europe.18,19 The aim of this study was to analyze the association between steady-state hemolysis assessed indirectly by a multivariable hemolysis index, anemia, and the main vascular complications of SCD in sub-Saharan Africa.
Patients and methods
This study was nested in the CADRE cohort, a multinational prospective observational study conducted in 5 sub-Saharan African countries (Cameroon, Senegal, Ivory Coast, Mali, Gabon). A detailed protocol of the study is available in Ranque et al.17 Only patients from Cameroon, Mali, and Ivory Coast were considered for the present study, because the hemolysis parameters were not available in Senegal or Gabon. SCD patients were recruited through outpatient clinics in 2 public and 1 private hospital in Yaoundé, 1 public hospital in Douala (Cameroon), 1 university hospital in Abidjan (Ivory Coast), and the Centre de Recherche et de Lutte contre la Drépanocytose in Bamako (Mali). The study was announced through poster, radio, and newspaper advertising to recruit patients that would not spontaneously seek medical care at the hospital. SCD patient associations were contacted and asked to encourage their members to participate in the study. Patients did not receive any financial incentive, but were offered reimbursement of transportation costs and free medical examination, biological analyses, and echocardiography. The list of CADRE investigators is provided in the supplemental Data, available on the Blood Web site. Patients were recruited from February 2011 to December 2013. Relevant national ethics committees in each of the participating countries approved the CADRE protocol: Ivory Coast (N043/MSLS/CNER-dkn), Mali (N18 MS-SG-CNESS/2011), and Cameroon (N016-CNE-SE-2011).
Inclusion criteria
All SCD patients aged 3 years and older were invited to participate in the study. The patients (or their parents) were required to sign an informed consent form to be included. The medical visits were performed at steady state (ie, at least 15 days after the last vaso-occlusive crisis [VOC], 8 days after fever or infectious disease, and 3 months after the last transfusion).
Data collection
Medical history, including the main clinical complications of SCD, and physical examination findings were recorded on standardized forms. Alkaline hemoglobin electrophoresis and/or high-performance liquid chromatography were performed to confirm the SCD phenotype (SS or sickle-β-zero-thalassemia [Sβ0], SC, sickle β+-thalassemia [Sβ+]). Fetal hemoglobin (HbF) levels were measured by high-performance liquid chromatography, when available. The SS and Sβ0 phenotypes can be misdiagnosed with this approach, and the clinical presentations of the 2 phenotypes are close.20 Thus, we chose to pool these patients together for the analysis. Other laboratory tests included complete blood counts, reticulocyte counts, bilirubin and LDH blood levels, adjusted for the normal upper range of each center, and serum and urine creatinine levels. Quantitative albuminuria was measured using 2 validated automated devices provided for the study: a Siemens Clinitek Status Analyzer (Erlangen, Germany) in Cameroon and Ivory Coast, and a Hemocue Albumin 20 system (Ängelholm, Sweden) in Mali. Microalbuminuria was defined as a urine albumin/creatinine ratio >30 mg/g. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease–Epidemiology Collaboration formula without adjusting for African ethnicity in adults21 and the Schwartz formula in children.22 A trained cardiologist performed the echocardiography, and measurements were recorded according to the recommendations of the American Society of Echocardiography.23 Tricuspid regurgitant jet velocity (TRV) was considered to be abnormally elevated for values >2.5 m/s.24,25
Imputations
LDH levels were not measured during the first 6 months of the study. Thus, 33% of LDH values were imputed using multiple imputations with full conditional specification (68% in Cameroon, 16% in Ivory Coast, and 16% in Mali). Detailed methods of imputation are described in the supplemental Data. The convergence of the imputation algorithm was assessed by Kendall plot and plots of the means and standard deviations per iteration for 3 imputed variables (supplemental Figures 1 and 2).26,27
Hemolysis assessment
We estimated the rate of hemolysis using a calculated index based on available clinical laboratory correlates. This index explained the maximum of the shared variance among hemolysis markers, based on the same statistical method but using different parameters than previously published.16 This was achieved by performing principal component analysis (PCA)28 using 2 common hemolysis markers: log-transformed LDH and a composite marker consisting of total bilirubin and clinical icterus. The bilirubin measurement was missing for 63% of patients. We therefore created a binary factor that was positive if the bilirubin level was >25 mg/L or if clinical icterus was present when bilirubin measurements were missing. The cutoff value of 25 mg/L was determined by receiver operating characteristic curve analysis using bilirubin as a predictor of clinical icterus (area under curve = 0.8). We used the first component coordinates of the 2 markers to calculate the index, and the resulting values were normalized to obtain the final index. This index can be best defined as an indirect hemolysis index because it was not validated against direct measurement of hemolysis.
Associations with SCD-related vascular complications
The characteristics of 3 phenotypic groups (SS-Sβ0, SC, and Sβ+) were compared using analysis of variance or the χ2 test, as appropriate. Patients with increased values of the indirect hemolysis index (defined for each phenotype as patients with an index value in the fourth quartile) were compared with the others (quartiles 1 to 3).
The principal analyses assessed the associations between SCD-related vascular complications and (a) a low hemoglobin level (in the first quartile) or (b) a high indirect hemolysis index (in the fourth quartile). These associations were assessed in the whole population and in each phenotypic group using logistic regression models, with adjustment for sex, country, age, phenotypic groups (in the whole population), and the type of microalbuminuria measurement device used for the analysis of albuminuria. We then adjusted the model involving the hemolysis index for hemoglobin level. SCD complications included elevated TRV, microalbuminuria, leg ulcers, priapism, osteonecrosis, and stroke.
We performed several sensitivity analyses. First, we compared the association between SCD-related vascular complications and the indirect hemolysis index in the adult population only (>18 years old), in children, and separately for each country. Second, we performed the same analyses using only LDH (imputed or not) as the surrogate of hemolysis. Third, we used the quantitative value instead of a binary categorization for the hemolysis index as well as for TRV and microalbuminuria.
A 2-sided P value <.05 was considered to be significant. All statistical analyses were performed using R software (version 3.1.1).
Results
Population description
Among the 3966 SCD patients included in the CADRE cohort, we excluded patients from Gabon (n = 540) and Senegal (n = 912) because of missing hemolysis data. Patients with no definitive SCD phenotype (n = 107) were also excluded from the analysis. Hence, 2407 patients were included in the present study, classified as follows: 1751 SS-Sβ0, 495 SC, and 161 Sβ+ phenotypes. The characteristics of the patients are described in Table 1. The distribution of SCD phenotypes varied by country (P < .001). The sex ratio was similar in the 4 phenotypic groups. SS-Sβ0 patients were younger than Sβ+ and SC patients: median (interquartile range, IQR), 15.0 (9.0-23.0) for SS-Sβ0 vs 21.0 (14.0-31.0) years old for SC. SS-Sβ0 patients presented with significantly more frequent VOCs, acute chest syndrome, leg ulcers, priapism, and osteonecrosis than Sβ+ or SC patients (P < .05). Less than 2% of SCD patients were receiving hydroxycarbamide or had iterative blood transfusions. At clinical examination, SS-Sβ0 patients had more frequent icterus than Sβ+ and SC patients (55.8% vs 13.8% and 8.9%, respectively, P < .001). SS-Sβ0 patients also had lower hemoglobin levels than Sβ+ and SC patients (median 7.9 g/dL vs 10.9 g/dL and 11.2 g/dL, respectively) and higher hemolysis marker levels (reticulocytes, LDH, and bilirubin). Renal explorations showed a higher rate of microalbuminuria and a higher eGFR in SS-Sβ0 patients than in SC and Sβ+ patients. Elevated TRV (>2.5 m/s) was diagnosed in 115 (24.8%) of the 431 SCD patients that underwent an echocardiography, without any significant difference between the 3 groups of patients.
Baseline characteristics of the study population classified by phenotype of SCD
| . | SS-Sβ0 . | SC . | Sβ+ . | . |
|---|---|---|---|---|
| Hemoglobin phenotype . | N = 1751 . | N = 495 . | N = 161 . | P* . |
| Country | <.001 | |||
| Cameroon, n (%) | 898 (51.3) | 3 (0.6) | 22 (13.7) | |
| Ivory Coast, n (%) | 333 (19.0) | 194 (39.2) | 94 (58.4) | |
| Mali, n (%) | 520 (29.7) | 298 (60.2) | 45 (28.0) | |
| Clinical findings | ||||
| Male sex, n (%) | 809 (46.2) | 218 (44.0) | 67 (41.6) | .503 |
| Age, y, median (IQR) | 15.0 (9.0-23.0) | 21.0 (14.0-31.0) | 18.0 (13.0-29.0) | <.001 |
| Body mass index, kg/m2, median (IQR) | 16.8 (14.4-19.6) | 19.4 (16.6-22.4) | 18.7 (16.2-21.3) | <.001 |
| Icterus, n (%) | 949 (55.8) | 43 (8.9) | 22 (13.8) | <.001 |
| Medical history | ||||
| Age of clinical onset, median (IQR) | 4.0 (1.0-8.0) | 12.0 (6.0-20.0) | 8.0 (4.0-16.8) | <.001 |
| More than 1 VOC last year, n (%) | 858 (50.2) | 212 (44.4) | 47 (30.3) | <.001 |
| More than 1 ACS lifetime, n (%) | 201 (12.0) | 23 (4.9) | 13 (8.5) | <.001 |
| Stroke, lifetime, n (%) | 23 (1.3) | 2 (0.4) | 0 (0.0) | .156 |
| Leg ulcer, lifetime, n (%) | 166 (9.5) | 18 (3.6) | 6 (3.7) | <.001 |
| Priapism, lifetime, n (%)† | 126 (15.6) | 30 (13.7) | 4 (6.0) | .007 |
| Osteonecrosis, lifetime, n (%) | 209 (11.9) | 49 (9.9) | 19 (11.8) | .044 |
| Treatment | ||||
| Hydroxycarbamide, n (%) | 27 (1.5) | 6 (1.2) | 1 (0.6) | .777 |
| Blood transfusion history, n (%) | 20 (1.1) | 4 (0.8) | 0 (0) | .571 |
| Echocardiographic parameters | ||||
| LVEF, %, median (IQR) | 64.0 (59.0-71.0) | 69.0 (64.0-68.0) | 69.0 (66.0-74.3) | <.001 |
| TRV >2.5 m/s, n (%) | 95 (28.4) | 16 (20.0) | 4 (25.0) | .127 |
| Biological findings | ||||
| Hemoglobin, g/dL, median (IQR) | 7.9 (7.0-8.9) | 11.2 (10.4-12.1) | 10.9 (9.6-11.9) | <.001 |
| Reticulocytes, 103/μL, median (IQR) | 201 (147-252) | 61.5 (8-120) | 22 (5-145) | <.001 |
| Bilirubin, µmol/L, median (IQR) | 27.0 (18.0-39.0) | 11.5 (8.0-18.0) | 11.0 (7.0-20.0) | <.001 |
| LDH, UI/L, median (IQR) | 822 (554-1246) | 391 (280-537) | 344 (243-598) | <.001 |
| HbF, %, median (IQR) | 8.3 (5.0-13.0) | 1.0 (0.0-3.0) | 6.0 (4.0-8.3) | <.001 |
| Leukocytes, G/L, median (IQR) | 11.6 (9.1-14.8) | 7.5 (5.8-9.3) | 5.9 (4.6-8.2) | <.001 |
| Platelets, G/L, median (IQR) | 401 (292-517) | 304 (230-401) | 275 (200-372) | <.001 |
| Urine ACR >30 mg/g, n (%) | 573 (42.4) | 93 (21.2) | 26 (17.0) | <.001 |
| eGFR mL/min/1.73 m2, median (IQR) | 194 (154-248) | 118 (102-136) | 123 (109-149) | <.001 |
| Hemolysis index, median (IQR) | 0.38 (−0.1:1.0) | −1.03 (−1.5:−0.6) | −1.17 (−1.6: −0.6) | <.001 |
| . | SS-Sβ0 . | SC . | Sβ+ . | . |
|---|---|---|---|---|
| Hemoglobin phenotype . | N = 1751 . | N = 495 . | N = 161 . | P* . |
| Country | <.001 | |||
| Cameroon, n (%) | 898 (51.3) | 3 (0.6) | 22 (13.7) | |
| Ivory Coast, n (%) | 333 (19.0) | 194 (39.2) | 94 (58.4) | |
| Mali, n (%) | 520 (29.7) | 298 (60.2) | 45 (28.0) | |
| Clinical findings | ||||
| Male sex, n (%) | 809 (46.2) | 218 (44.0) | 67 (41.6) | .503 |
| Age, y, median (IQR) | 15.0 (9.0-23.0) | 21.0 (14.0-31.0) | 18.0 (13.0-29.0) | <.001 |
| Body mass index, kg/m2, median (IQR) | 16.8 (14.4-19.6) | 19.4 (16.6-22.4) | 18.7 (16.2-21.3) | <.001 |
| Icterus, n (%) | 949 (55.8) | 43 (8.9) | 22 (13.8) | <.001 |
| Medical history | ||||
| Age of clinical onset, median (IQR) | 4.0 (1.0-8.0) | 12.0 (6.0-20.0) | 8.0 (4.0-16.8) | <.001 |
| More than 1 VOC last year, n (%) | 858 (50.2) | 212 (44.4) | 47 (30.3) | <.001 |
| More than 1 ACS lifetime, n (%) | 201 (12.0) | 23 (4.9) | 13 (8.5) | <.001 |
| Stroke, lifetime, n (%) | 23 (1.3) | 2 (0.4) | 0 (0.0) | .156 |
| Leg ulcer, lifetime, n (%) | 166 (9.5) | 18 (3.6) | 6 (3.7) | <.001 |
| Priapism, lifetime, n (%)† | 126 (15.6) | 30 (13.7) | 4 (6.0) | .007 |
| Osteonecrosis, lifetime, n (%) | 209 (11.9) | 49 (9.9) | 19 (11.8) | .044 |
| Treatment | ||||
| Hydroxycarbamide, n (%) | 27 (1.5) | 6 (1.2) | 1 (0.6) | .777 |
| Blood transfusion history, n (%) | 20 (1.1) | 4 (0.8) | 0 (0) | .571 |
| Echocardiographic parameters | ||||
| LVEF, %, median (IQR) | 64.0 (59.0-71.0) | 69.0 (64.0-68.0) | 69.0 (66.0-74.3) | <.001 |
| TRV >2.5 m/s, n (%) | 95 (28.4) | 16 (20.0) | 4 (25.0) | .127 |
| Biological findings | ||||
| Hemoglobin, g/dL, median (IQR) | 7.9 (7.0-8.9) | 11.2 (10.4-12.1) | 10.9 (9.6-11.9) | <.001 |
| Reticulocytes, 103/μL, median (IQR) | 201 (147-252) | 61.5 (8-120) | 22 (5-145) | <.001 |
| Bilirubin, µmol/L, median (IQR) | 27.0 (18.0-39.0) | 11.5 (8.0-18.0) | 11.0 (7.0-20.0) | <.001 |
| LDH, UI/L, median (IQR) | 822 (554-1246) | 391 (280-537) | 344 (243-598) | <.001 |
| HbF, %, median (IQR) | 8.3 (5.0-13.0) | 1.0 (0.0-3.0) | 6.0 (4.0-8.3) | <.001 |
| Leukocytes, G/L, median (IQR) | 11.6 (9.1-14.8) | 7.5 (5.8-9.3) | 5.9 (4.6-8.2) | <.001 |
| Platelets, G/L, median (IQR) | 401 (292-517) | 304 (230-401) | 275 (200-372) | <.001 |
| Urine ACR >30 mg/g, n (%) | 573 (42.4) | 93 (21.2) | 26 (17.0) | <.001 |
| eGFR mL/min/1.73 m2, median (IQR) | 194 (154-248) | 118 (102-136) | 123 (109-149) | <.001 |
| Hemolysis index, median (IQR) | 0.38 (−0.1:1.0) | −1.03 (−1.5:−0.6) | −1.17 (−1.6: −0.6) | <.001 |
ACR, albumin/creatinine ratio; ACS, acute chest syndrome; LVEF, left ventricular ejection fraction; MCV, mean corpuscular volume; n, number; NA, not applicable.
P, bivariate comparison, analysis of variance for continuous variables, and χ2 test for discontinuous variables.
Percentages calculated for men and boys only.
Association between the degree of anemia and SCD complications
The lowest quartile of hemoglobin was significantly associated with elevated TRV and microalbuminuria independently of age, sex, country, or SCD phenotype in the whole population (Table 2). We also performed multivariate analyses separately on the adult and pediatric populations (Table 3). The adult SS-Sβ0 population (aged 18 years and older) had the highest associations between low hemoglobin and elevated TRV (odds ratio [OR] = 3.22 [1.48-7.00]) and microalbuminuria (OR = 2.05 [1.27-3.31]). There was also a significant association with leg ulcers (OR = 1.76 [1.11-2.79]). In the pediatric population, only the association with microalbuminuria (OR = 1.47 [1.05-2.06]) was significant.
Associations between SCD-related vascular complications and hemoglobin (quartile 1 vs quartiles 2 to 4 of hemoglobin) in the overall sample, SS-Sβ0, SC, and Sβ+patients: multivariate analysis
| . | . | % of patients with the complication depending on Hb level . | Multivariate analysis* . | |||
|---|---|---|---|---|---|---|
| . | N event/N total . | Quartile 1 . | Quartiles 2 to 4 . | OR . | CI 95% . | P* . |
| Overall sample | ||||||
| TRV >2.5 m/s† | 115/431 | 38.0 | 24.1 | 1.86 | 1.04-3.32 | .035 |
| TRV >3 m/s | 9/431 | 6.5 | 1.0 | 8.94 | 1.54-51.93 | .015 |
| Urine ACR >30 mg/g‡ | 724/1991 | 51.5 | 30.8 | 1.74 | 1.34-2.24 | <.001 |
| Leg ulcer, lifetime | 190/2409 | 9.8 | 7.4 | 1.17 | 0.81-1.71 | .406 |
| Priapism, lifetime§ | 160/1095 | 16.6 | 13.6 | 1.51 | 0.99-2.32 | .057 |
| Stroke, lifetime | 25/2409 | 1.1 | 1.1 | 0.65 | 0.25-1.72 | .387 |
| Osteonecrosis, lifetime | 277/2409 | 10.5 | 11.6 | 0.84 | 0.60-1.19 | .334 |
| SS-Sβ0phenotype | ||||||
| TRV >2.5 m/s† | 95/335 | 41.9 | 24.9 | 2.26 | 1.22-4.17 | .009 |
| TRV >3 m/s | 8/335 | 6.8 | 1.3 | 6.66 | 1.30-34.16 | .023 |
| Urine ACR >30 mg/g‡ | 573/1351 | 52.8 | 38.9 | 1.48 | 1.12-1.95 | .006 |
| Leg ulcer, lifetime | 166/1751 | 10.8 | 9.2 | 1.20 | 0.80-1.78 | .373 |
| Priapism, lifetime§ | 126/809 | 14.8 | 15.4 | 1.11 | 0.69-1.79 | .664 |
| Stroke, lifetime | 23/1751 | 1.2 | 1.4 | 0.66 | 0.24-1.83 | .425 |
| Osteonecrosis, lifetime | 209/1751 | 12.2 | 11.5 | 1.01 | 0.70-1.46 | .948 |
| SC phenotype | ||||||
| TRV >2.5 m/s† | 16/80 | 30.8 | 18.3 | 2.32 | 0.55-9.76 | .005 |
| TRV >3 m/s | 1/80 | 0.0 | 1.7 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 93/339 | 28.9 | 18.6 | 1.85 | 1.06-3.22 | .030 |
| Leg ulcer, lifetime | 18/495 | 4.6 | 3.2 | 1.79 | 0.59-5.42 | .299 |
| Priapism, lifetime§ | 30/218 | 7.1 | 15.4 | 0.46 | 0.13-1.71 | .246 |
| Stroke, lifetime | 2/495 | 0.0 | 0.5 | NA | NA | NA |
| Osteonecrosis, lifetime | 49/495 | 7.3 | 10.6 | 0.84 | 0.37-1.93 | .686 |
| Sβ+ phenotype | ||||||
| TRV >2.5 m/s | 4/16 | 40.0 | 20.0 | 3.97 | 0.17-94.93 | .353 |
| TRV >3 m/s | 0/16 | 0.0 | 0.0 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 26/153 | 28.9 | 13.3 | 1.27 | 0.37-4.33 | .689 |
| Leg ulcer, lifetime | 6/161 | 7.9 | 2.5 | 1.58 | 0.14-17.86 | .711 |
| Priapism, lifetime§ | 4/67 | 5.3 | 1.7 | 0.77 | 0.02-32.13 | .891 |
| Stroke, lifetime | 0/161 | 0.0 | 0.0 | NA | NA | NA |
| Osteonecrosis, lifetime | 19/161 | 10.5 | 12.4 | 0.77 | 0.13-4.75 | .782 |
| . | . | % of patients with the complication depending on Hb level . | Multivariate analysis* . | |||
|---|---|---|---|---|---|---|
| . | N event/N total . | Quartile 1 . | Quartiles 2 to 4 . | OR . | CI 95% . | P* . |
| Overall sample | ||||||
| TRV >2.5 m/s† | 115/431 | 38.0 | 24.1 | 1.86 | 1.04-3.32 | .035 |
| TRV >3 m/s | 9/431 | 6.5 | 1.0 | 8.94 | 1.54-51.93 | .015 |
| Urine ACR >30 mg/g‡ | 724/1991 | 51.5 | 30.8 | 1.74 | 1.34-2.24 | <.001 |
| Leg ulcer, lifetime | 190/2409 | 9.8 | 7.4 | 1.17 | 0.81-1.71 | .406 |
| Priapism, lifetime§ | 160/1095 | 16.6 | 13.6 | 1.51 | 0.99-2.32 | .057 |
| Stroke, lifetime | 25/2409 | 1.1 | 1.1 | 0.65 | 0.25-1.72 | .387 |
| Osteonecrosis, lifetime | 277/2409 | 10.5 | 11.6 | 0.84 | 0.60-1.19 | .334 |
| SS-Sβ0phenotype | ||||||
| TRV >2.5 m/s† | 95/335 | 41.9 | 24.9 | 2.26 | 1.22-4.17 | .009 |
| TRV >3 m/s | 8/335 | 6.8 | 1.3 | 6.66 | 1.30-34.16 | .023 |
| Urine ACR >30 mg/g‡ | 573/1351 | 52.8 | 38.9 | 1.48 | 1.12-1.95 | .006 |
| Leg ulcer, lifetime | 166/1751 | 10.8 | 9.2 | 1.20 | 0.80-1.78 | .373 |
| Priapism, lifetime§ | 126/809 | 14.8 | 15.4 | 1.11 | 0.69-1.79 | .664 |
| Stroke, lifetime | 23/1751 | 1.2 | 1.4 | 0.66 | 0.24-1.83 | .425 |
| Osteonecrosis, lifetime | 209/1751 | 12.2 | 11.5 | 1.01 | 0.70-1.46 | .948 |
| SC phenotype | ||||||
| TRV >2.5 m/s† | 16/80 | 30.8 | 18.3 | 2.32 | 0.55-9.76 | .005 |
| TRV >3 m/s | 1/80 | 0.0 | 1.7 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 93/339 | 28.9 | 18.6 | 1.85 | 1.06-3.22 | .030 |
| Leg ulcer, lifetime | 18/495 | 4.6 | 3.2 | 1.79 | 0.59-5.42 | .299 |
| Priapism, lifetime§ | 30/218 | 7.1 | 15.4 | 0.46 | 0.13-1.71 | .246 |
| Stroke, lifetime | 2/495 | 0.0 | 0.5 | NA | NA | NA |
| Osteonecrosis, lifetime | 49/495 | 7.3 | 10.6 | 0.84 | 0.37-1.93 | .686 |
| Sβ+ phenotype | ||||||
| TRV >2.5 m/s | 4/16 | 40.0 | 20.0 | 3.97 | 0.17-94.93 | .353 |
| TRV >3 m/s | 0/16 | 0.0 | 0.0 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 26/153 | 28.9 | 13.3 | 1.27 | 0.37-4.33 | .689 |
| Leg ulcer, lifetime | 6/161 | 7.9 | 2.5 | 1.58 | 0.14-17.86 | .711 |
| Priapism, lifetime§ | 4/67 | 5.3 | 1.7 | 0.77 | 0.02-32.13 | .891 |
| Stroke, lifetime | 0/161 | 0.0 | 0.0 | NA | NA | NA |
| Osteonecrosis, lifetime | 19/161 | 10.5 | 12.4 | 0.77 | 0.13-4.75 | .782 |
CI, confidence interval; Hb, hemoglobin.
Adjustment for age, sex, country, plus SCD phenotype for the overall sample.
Percentages calculated for patients from Mali and Cameroon only.
Additional adjustment for the laboratory technique used.
Percentages calculated for males only.
Associations between SCD-related vascular complications and hemoglobin (quartile 1 vs quartiles 2 to 4 of hemoglobin): multivariate analysis in adult and pediatric SS or Sβ0 populations
| . | . | % of patients with the complication depending on Hb level . | Multivariate analysis* . | |||
|---|---|---|---|---|---|---|
| Complication, N (%) . | N event/N total . | Quartile 1 . | Quartiles 2 to 4 . | OR . | 95% CI . | P* . |
| Adult SS or Sβ0phenotype population (≥18 y old, n = 710) | ||||||
| TRV >2.5 m/s† | 69/223 | 55.3 | 27.2 | 3.22 | 1.48-7.00 | .003 |
| TRV >3m/s | 6/223 | 10.5 | 1.2 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 249/557 | 59.8 | 41.0 | 2.05 | 1.27-3.31 | .003 |
| Leg ulcer, lifetime | 128/710 | 25.2 | 16.8 | 1.76 | 1.11-2.79 | .017 |
| Priapism, lifetime§ | 81/306 | 33.3 | 24.0 | 1.66 | 0.84-3.27 | .144 |
| Stroke, lifetime | 13/710 | 2.3 | 1.8 | 1.27 | 0.34-4.70 | .721 |
| Osteonecrosis, lifetime | 136/710 | 18.3 | 18.8 | 0.99 | 0.60-1.62 | .957 |
| Pediatric SS or Sβ0phenotype population (<18 y old, n = 1041) | ||||||
| TRV >2.5 m/s† | 26/112 | 27.8 | 19.1 | 1.67 | 0.63-4.43 | .297 |
| TRV >3 m/s† | 2/112 | 2.8 | 1.5 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 324/794 | 49.8 | 37.3 | 1.47 | 1.05-2.06 | .003 |
| Leg ulcer, lifetime | 38/1041 | 4.2 | 3.5 | 1.28 | 0.63-2.62 | .493 |
| Priapism, lifetime§ | 45/503 | 9.1 | 9.0 | 0.84 | 0.43-1.67 | .625 |
| Stroke, lifetime | 10/1041 | 0.7 | 1.1 | 0.49 | 0.10-2.42 | .381 |
| Osteonecrosis, lifetime | 73/1041 | 9.4 | 6.1 | 1.25 | 0.73-2.15 | .408 |
| . | . | % of patients with the complication depending on Hb level . | Multivariate analysis* . | |||
|---|---|---|---|---|---|---|
| Complication, N (%) . | N event/N total . | Quartile 1 . | Quartiles 2 to 4 . | OR . | 95% CI . | P* . |
| Adult SS or Sβ0phenotype population (≥18 y old, n = 710) | ||||||
| TRV >2.5 m/s† | 69/223 | 55.3 | 27.2 | 3.22 | 1.48-7.00 | .003 |
| TRV >3m/s | 6/223 | 10.5 | 1.2 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 249/557 | 59.8 | 41.0 | 2.05 | 1.27-3.31 | .003 |
| Leg ulcer, lifetime | 128/710 | 25.2 | 16.8 | 1.76 | 1.11-2.79 | .017 |
| Priapism, lifetime§ | 81/306 | 33.3 | 24.0 | 1.66 | 0.84-3.27 | .144 |
| Stroke, lifetime | 13/710 | 2.3 | 1.8 | 1.27 | 0.34-4.70 | .721 |
| Osteonecrosis, lifetime | 136/710 | 18.3 | 18.8 | 0.99 | 0.60-1.62 | .957 |
| Pediatric SS or Sβ0phenotype population (<18 y old, n = 1041) | ||||||
| TRV >2.5 m/s† | 26/112 | 27.8 | 19.1 | 1.67 | 0.63-4.43 | .297 |
| TRV >3 m/s† | 2/112 | 2.8 | 1.5 | NA | NA | NA |
| Urine ACR >30 mg/g‡ | 324/794 | 49.8 | 37.3 | 1.47 | 1.05-2.06 | .003 |
| Leg ulcer, lifetime | 38/1041 | 4.2 | 3.5 | 1.28 | 0.63-2.62 | .493 |
| Priapism, lifetime§ | 45/503 | 9.1 | 9.0 | 0.84 | 0.43-1.67 | .625 |
| Stroke, lifetime | 10/1041 | 0.7 | 1.1 | 0.49 | 0.10-2.42 | .381 |
| Osteonecrosis, lifetime | 73/1041 | 9.4 | 6.1 | 1.25 | 0.73-2.15 | .408 |
Adjustment for age, sex, country, plus SCD phenotype for the overall sample.
Percentages calculated for patients from Mali and Cameroon only.
Additional adjustment for the laboratory technique used.
Percentages calculated for males only.
Indirect hemolysis index
The indirect hemolysis index derived from the first component of the PCA accounted for 66.3% of total variance of the 2 hemolysis markers (LDH and icterus) and had an Eigenvalue of 1.3.
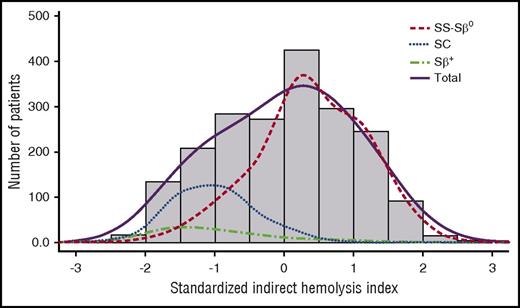
Consistent with the known differences in hemolytic intensity among the various SCD genotypes, the hemolysis index was significantly different between the 4 groups, with a median index of 0.38 for SS-Sβ0 patients, −1.03 for SC patients and −1.17 for Sβ+ patients, P < .001 (Figure 1).
Distribution of the standardized indirect hemolysis index in the overall sample and in SS-Sβ0, SC, and Sβ+patients. Bar charts indicate the observed sample size in each 0.5 level class of hemolysis index. Lines indicate the smoothed distributions in the overall sample (solid line) or in different phenotypic groups (dashed lines).
Distribution of the standardized indirect hemolysis index in the overall sample and in SS-Sβ0, SC, and Sβ+patients. Bar charts indicate the observed sample size in each 0.5 level class of hemolysis index. Lines indicate the smoothed distributions in the overall sample (solid line) or in different phenotypic groups (dashed lines).
Association between a high indirect hemolysis index and SCD complications
Univariate analysis of the whole population showed that the quartile of patients with the highest hemolysis index (quartile 4) had a higher prevalence of elevated microalbuminuria, leg ulcers (P < .001), priapism, osteonecrosis (P < .05), stroke (P = .055), and elevated TRV (P = .087). After adjustment for age, sex, country, and SCD phenotype, the indirect hemolysis index remained significantly associated with microalbuminuria (OR = 1.62, 95% CI [1.19 to 2.21]) only (Table 4). The results were similar when the quantitative (instead of binary) values of TRV and microalbuminuria were used (P values of .212 and .001, respectively).
Associations between SCD-related vascular complications and the indirect hemolysis index (quartiles 1 to 3 vs quartile 4 of the hemolysis index) in the overall sample, SS-Sβ0, SC, and Sβ+ patients: multivariate analysis
| . | . | % of patients with the complication depending on hemolysis index . | Multivariate analysis* . | . | |||
|---|---|---|---|---|---|---|---|
| . | N event/N total . | Quartile 1 to 3 . | Quartile 4 . | OR . | CI 95% . | P* . | P† . |
| Overall sample | |||||||
| TRV >2.5 m/s‡ | 115/431 | 24.0 | 33.1 | 1.40 | 0.78-2.51 | .262 | .372 |
| TRV >3 m/s‡ | 9/431 | 1.2 | 4.3 | 6.06 | 0.65-56.16 | .112 | .226 |
| Urine ACR >30 mg/g§ | 724/1991 | 29.4 | 54.8 | 1.62 | 1.19-2.21 | .003 | .106 |
| Leg ulcer, lifetime | 190/2409 | 6.4 | 12.3 | 1.53 | 0.96-2.43 | .071 | .199 |
| Priapism, lifetime‖ | 160/1095 | 13.1 | 18.4 | 1.49 | 0.95-2.33 | .083 | .267 |
| Stroke, lifetime | 25/2409 | 0.8 | 1.8 | 1.33 | 0.49-3.62 | .580 | .747 |
| Osteonecrosis, lifetime | 277/2409 | 10.6 | 14.0 | 1.22 | 0.85-1.76 | .274 | .240 |
| SS-Sβ0phenotype | |||||||
| TRV >2.5 m/s‡ | 95/335 | 25.2 | 37.8 | 1.71 | 0.86-3.40 | .127 | .226 |
| TRV >3 m/s‡ | 8/335 | 1.8 | 4.1 | 3.51 | 0.40-30.6 | .252 | .668 |
| Urine ACR >30 mg/g§ | 573/1351 | 37.5 | 56.3 | 1.42 | 1.03-1.95 | .034 | .225 |
| Leg ulcer, lifetime | 166/1751 | 8.3 | 12.9 | 1.50 | 0.93-2.44 | .096 | .098 |
| Priapism, lifetime‖ | 126/809 | 14.6 | 17.9 | 1.32 | 0.80-2.16 | .280 | .586 |
| Stroke, lifetime | 23/1751 | 1.2 | 1.7 | 1.01 | 0.33-3.04 | .991 | .920 |
| Osteonecrosis, lifetime | 209/1751 | 11.0 | 14.5 | 1.25 | 0.82-1.89 | .302 | .167 |
| SC phenotype | |||||||
| TRV >2.5 m/s‡ | 16/80 | 20.7 | 49.3 | 1.30 | 0.34-5.00 | .695 | .880 |
| TRV >3 m/s‡ | 1/80 | 1.70 | 0.0 | NA | NA | NA | NA |
| Urine ACR >30 mg/g§ | 93/339 | 20.7 | 22.2 | 0.82 | 0.46-1.47 | .504 | .269 |
| Leg ulcer, lifetime | 18/495 | 3.7 | 3.3 | 0.70 | 0.22-2.26 | .549 | .423 |
| Priapism, lifetime‖ | 30/218 | 13.8 | 13.5 | 0.85 | 0.35-2.07 | .717 | .867 |
| Stroke, lifetime | 2/495 | 0.54 | 0.0 | NA | NA | NA | NA |
| Osteonecrosis, lifetime | 49/495 | 9.9 | 9.9 | 0.95 | 0.45-2.03 | .899 | .960 |
| Sβ+ phenotype | |||||||
| TRV >2.5 m/s‡ | 4/16 | 13.7 | 26.7 | 1.37 | 0.05-34.5 | .831 | .384 |
| TRV >3 m/s‡ | 0/16 | 0.0 | 0.0 | NA | NA | NA | NA |
| Urine ACR >30 mg/g§ | 26/153 | 13.6 | 26.7 | 0.86 | 0.22-3.42 | .835 | .470 |
| Leg ulcer, lifetime | 6/161 | 1.6 | 9.9 | 4.52 | 0.35-58.81 | .247 | .920 |
| Priapism, lifetime‖ | 4/67 | 4.3 | 9.8 | 0.27 | 0.01-8.42 | .456 | .433 |
| Stroke, lifetime | 0/161 | 0.0 | 0.0 | NA | NA | NA | NA |
| Osteonecrosis, lifetime | 19/161 | 13.4 | 7.3 | 0.15 | 0.01-2.49 | .186 | .225 |
| . | . | % of patients with the complication depending on hemolysis index . | Multivariate analysis* . | . | |||
|---|---|---|---|---|---|---|---|
| . | N event/N total . | Quartile 1 to 3 . | Quartile 4 . | OR . | CI 95% . | P* . | P† . |
| Overall sample | |||||||
| TRV >2.5 m/s‡ | 115/431 | 24.0 | 33.1 | 1.40 | 0.78-2.51 | .262 | .372 |
| TRV >3 m/s‡ | 9/431 | 1.2 | 4.3 | 6.06 | 0.65-56.16 | .112 | .226 |
| Urine ACR >30 mg/g§ | 724/1991 | 29.4 | 54.8 | 1.62 | 1.19-2.21 | .003 | .106 |
| Leg ulcer, lifetime | 190/2409 | 6.4 | 12.3 | 1.53 | 0.96-2.43 | .071 | .199 |
| Priapism, lifetime‖ | 160/1095 | 13.1 | 18.4 | 1.49 | 0.95-2.33 | .083 | .267 |
| Stroke, lifetime | 25/2409 | 0.8 | 1.8 | 1.33 | 0.49-3.62 | .580 | .747 |
| Osteonecrosis, lifetime | 277/2409 | 10.6 | 14.0 | 1.22 | 0.85-1.76 | .274 | .240 |
| SS-Sβ0phenotype | |||||||
| TRV >2.5 m/s‡ | 95/335 | 25.2 | 37.8 | 1.71 | 0.86-3.40 | .127 | .226 |
| TRV >3 m/s‡ | 8/335 | 1.8 | 4.1 | 3.51 | 0.40-30.6 | .252 | .668 |
| Urine ACR >30 mg/g§ | 573/1351 | 37.5 | 56.3 | 1.42 | 1.03-1.95 | .034 | .225 |
| Leg ulcer, lifetime | 166/1751 | 8.3 | 12.9 | 1.50 | 0.93-2.44 | .096 | .098 |
| Priapism, lifetime‖ | 126/809 | 14.6 | 17.9 | 1.32 | 0.80-2.16 | .280 | .586 |
| Stroke, lifetime | 23/1751 | 1.2 | 1.7 | 1.01 | 0.33-3.04 | .991 | .920 |
| Osteonecrosis, lifetime | 209/1751 | 11.0 | 14.5 | 1.25 | 0.82-1.89 | .302 | .167 |
| SC phenotype | |||||||
| TRV >2.5 m/s‡ | 16/80 | 20.7 | 49.3 | 1.30 | 0.34-5.00 | .695 | .880 |
| TRV >3 m/s‡ | 1/80 | 1.70 | 0.0 | NA | NA | NA | NA |
| Urine ACR >30 mg/g§ | 93/339 | 20.7 | 22.2 | 0.82 | 0.46-1.47 | .504 | .269 |
| Leg ulcer, lifetime | 18/495 | 3.7 | 3.3 | 0.70 | 0.22-2.26 | .549 | .423 |
| Priapism, lifetime‖ | 30/218 | 13.8 | 13.5 | 0.85 | 0.35-2.07 | .717 | .867 |
| Stroke, lifetime | 2/495 | 0.54 | 0.0 | NA | NA | NA | NA |
| Osteonecrosis, lifetime | 49/495 | 9.9 | 9.9 | 0.95 | 0.45-2.03 | .899 | .960 |
| Sβ+ phenotype | |||||||
| TRV >2.5 m/s‡ | 4/16 | 13.7 | 26.7 | 1.37 | 0.05-34.5 | .831 | .384 |
| TRV >3 m/s‡ | 0/16 | 0.0 | 0.0 | NA | NA | NA | NA |
| Urine ACR >30 mg/g§ | 26/153 | 13.6 | 26.7 | 0.86 | 0.22-3.42 | .835 | .470 |
| Leg ulcer, lifetime | 6/161 | 1.6 | 9.9 | 4.52 | 0.35-58.81 | .247 | .920 |
| Priapism, lifetime‖ | 4/67 | 4.3 | 9.8 | 0.27 | 0.01-8.42 | .456 | .433 |
| Stroke, lifetime | 0/161 | 0.0 | 0.0 | NA | NA | NA | NA |
| Osteonecrosis, lifetime | 19/161 | 13.4 | 7.3 | 0.15 | 0.01-2.49 | .186 | .225 |
Adjustment for age, sex, country, plus SCD phenotype for the overall sample.
Plus adjustment for Hb.
Percentages calculated for patients from Mali and Cameroon only.
Additional adjustment for the laboratory technique used.
Percentages calculated for males only.
The associations between the indirect hemolysis index and clinical complications were no longer significant after further adjustment for hemoglobin level (Table 4).
We performed a sensitivity analysis by restricting the above analyses to the SS-Sβ0 phenotype subgroup. The highest quartile of the hemolysis index remained significantly associated with microalbuminuria (OR = 1.42 [1.03-1.95]). Associations between the hemolysis index and vascular complications of SCD for SC and Sβ+ patients were not statistically significant, and the ORs were <1 for all complications, except elevated TRV in SC and Sβ+ patients and leg ulcers in Sβ+ patients (Table 4).
Multivariate analyses of the adult SS-Sβ0 population (Table 5) showed that the elevated indirect hemolysis index was associated with microalbuminuria (OR = 1.80 [1.10-2.95]) and leg ulcers (OR = 2.11 [1.22-3.62]). In SS-Sβ0 children (Table 3), an elevated index was associated with microalbuminuria only. These findings were similar to those of each country, determined separately (supplemental Table 1.
Associations between SCD-related vascular complications and the indirect hemolysis index (quartile 1 to 3 vs quartile 4 of the hemolysis index): multivariate analysis in adult and pediatric SS or Sβ0 populations
| . | . | % of patients with the complication depending on hemolysis index . | Multivariate analysis* . | ||||
|---|---|---|---|---|---|---|---|
| Complication, N (%) . | N event/N total . | Quartiles 1 to 3 . | Quartile 4 . | OR . | 95% CI . | P* . | P† . |
| Adult SS or Sβ0phenotype population (≥18 y old, n = 710) | |||||||
| TRV >2.5 m/s‡ | 69/223 | 27.4 | 45.2 | 2.17 | 0.92-5.14 | .078 | .374 |
| TRV >3 m/s‡ | 6/223 | 2.4 | 3.7 | 2.17 | 0.92-5.14 | .078 | .374 |
| Urine ACR >30 mg/g§ | 249/557 | 41.2 | 60.5 | 1.80 | 1.10-2.95 | .019 | .425 |
| Leg ulcer, lifetime | 128/710 | 15.5 | 28.2 | 2.11 | 1.22-3.62 | .007 | .059 |
| Priapism, lifetime‖ | 81/306 | 24.6 | 32.9 | 1.62 | 0.80-3.29 | .181 | .719 |
| Stroke, lifetime | 13/710 | 1.36 | 3.83 | 2.86 | 0.78-10.5 | .112 | .329 |
| Osteonecrosis, lifetime | 136/710 | 18.3 | 22.5 | 1.30 | 0.76-2.25 | .337 | .307 |
| Pediatric SS or Sβ0phenotype population (<18 y old, n = 1041) | |||||||
| TRV >2.5 m/s‡ | 26/112 | 19.7 | 29.4 | 1.69 | 0.62-4.58 | .299 | .158 |
| TRV >3 m/s‡ | 2/112 | 0.2 | 4.6 | 1.69 | 0.62-4.58 | .299 | .158 |
| Urine ACR >30 mg/g§ | 324/794 | 34.5 | 54.5 | 1.49 | 1.00-2.21 | .046 | .156 |
| Leg ulcer, lifetime | 38/1041 | 2.6 | 6.1 | 2.19 | 0.97-4.93 | .058 | .041 |
| Priapism, lifetime‖ | 45/503 | 7.6 | 11.7 | 1.17 | 0.57-2.41 | .665 | .751 |
| Stroke, lifetime | 10/1041 | 1.0 | 0.8 | NA | NA | NA | NA |
| Osteonecrosis, lifetime | 73/1041 | 5.3 | 11.0 | 1.30 | 0.73-2.33 | .367 | .368 |
| . | . | % of patients with the complication depending on hemolysis index . | Multivariate analysis* . | ||||
|---|---|---|---|---|---|---|---|
| Complication, N (%) . | N event/N total . | Quartiles 1 to 3 . | Quartile 4 . | OR . | 95% CI . | P* . | P† . |
| Adult SS or Sβ0phenotype population (≥18 y old, n = 710) | |||||||
| TRV >2.5 m/s‡ | 69/223 | 27.4 | 45.2 | 2.17 | 0.92-5.14 | .078 | .374 |
| TRV >3 m/s‡ | 6/223 | 2.4 | 3.7 | 2.17 | 0.92-5.14 | .078 | .374 |
| Urine ACR >30 mg/g§ | 249/557 | 41.2 | 60.5 | 1.80 | 1.10-2.95 | .019 | .425 |
| Leg ulcer, lifetime | 128/710 | 15.5 | 28.2 | 2.11 | 1.22-3.62 | .007 | .059 |
| Priapism, lifetime‖ | 81/306 | 24.6 | 32.9 | 1.62 | 0.80-3.29 | .181 | .719 |
| Stroke, lifetime | 13/710 | 1.36 | 3.83 | 2.86 | 0.78-10.5 | .112 | .329 |
| Osteonecrosis, lifetime | 136/710 | 18.3 | 22.5 | 1.30 | 0.76-2.25 | .337 | .307 |
| Pediatric SS or Sβ0phenotype population (<18 y old, n = 1041) | |||||||
| TRV >2.5 m/s‡ | 26/112 | 19.7 | 29.4 | 1.69 | 0.62-4.58 | .299 | .158 |
| TRV >3 m/s‡ | 2/112 | 0.2 | 4.6 | 1.69 | 0.62-4.58 | .299 | .158 |
| Urine ACR >30 mg/g§ | 324/794 | 34.5 | 54.5 | 1.49 | 1.00-2.21 | .046 | .156 |
| Leg ulcer, lifetime | 38/1041 | 2.6 | 6.1 | 2.19 | 0.97-4.93 | .058 | .041 |
| Priapism, lifetime‖ | 45/503 | 7.6 | 11.7 | 1.17 | 0.57-2.41 | .665 | .751 |
| Stroke, lifetime | 10/1041 | 1.0 | 0.8 | NA | NA | NA | NA |
| Osteonecrosis, lifetime | 73/1041 | 5.3 | 11.0 | 1.30 | 0.73-2.33 | .367 | .368 |
Adjustment for sex, country, and SCD phenotype.
Plus adjustment for Hb.
Percentages calculated for patients from Mali and Cameroon only.
Additional adjustment for the laboratory technique used.
Percentages calculated for males only.
Multivariate analysis of the association between clinical complications and the quantitative values of the hemolysis index yielded similar results as those with the highest quartile (supplemental Table 2).
If LDH was used as the sole hemolysis marker, there was a significant association of the highest quartile of LDH with microalbuminuria in SS-Sβ0 patients only (supplemental Table 3a). These associations were similar when using directly measured (nonimputed) LDH values only (n = 1594 patients; supplemental Table 3b). In the SC and Sβ+ phenotypic groups, associations between increased hemolysis, defined by either the imputed or the nonimputed fourth quartile of LDH, and SCD-related vascular complications were not significant (supplemental Table S3).
Discussion
In this study, conducted for the first time in a large cohort of SCD patients living in West and Central Africa, we observed a significant association between the degree of anemia at steady state and elevated TRV and microalbuminuria. Low hemoglobin levels were also associated with leg ulcers in SS-Sβ0 adults. The indirect hemolysis index we used was weakly, although significantly, associated with microalbuminuria and elevated TRV in the whole population and leg ulcers in adult SS and Sβ0 patients. However, we did not consistently observe these associations in children and or other SCD phenotypes and, strikingly, they did not remain significant after adjustment for hemoglobin level. Moreover, there was no significant association between the indirect hemolysis index and stroke or priapism, irrespective of the SCD phenotype.
Several studies have analyzed these associations in SCD populations living outside Africa. Gladwin et al25 first demonstrated an association between Doppler-defined pulmonary hypertension (TRV > 2.5 m/s) and LDH values (OR = 2.7 [1.3-5.9]) in 149 African American SCD patients in a prospective screening study at the National Institutes of Health. In the same cohort, Kato et al29 showed an association between LDH values and elevated TRV, leg ulcers, and priapism in 213 SCD patients. Taylor et al analyzed the effect of increased LDH (fourth quartile) in 350 adult African American (mean age 36.3 years old) SCD patients. Increased LDH was strongly associated with leg ulcers (OR = 3.27), priapism (OR = 2.62), and pulmonary hypertension, defined by NT-proBNP (OR = 4.32).10 No association was noted with stroke, and microalbuminuria was not investigated. The Walk-PHASST study analyzed the clinical correlates of a composite component, including various hemolytic markers (LDH, bilirubin, aspartate aminotransferase [ASAT], and reticulocyte count), in 415 African American or British SS patients.15 The authors found an association between the hemolytic component and TRV in the whole population and with leg ulcers in the subgroup of patients not treated with hydroxycarbamide. No association with chronic renal failure or osteonecrosis was found. Stroke and microalbuminuria were not investigated. Finally, Minniti et al conducted a prospective pediatric study of American SCD patients specifically focused on elevated TRV and found an association with an increase of the same composite hemolytic component (OR = 4.5 [1.5-14.4]).16 Other studies have observed an association between hemolytic markers and leg ulcers,30-33 priapism,34 stroke,35,36 and microalbuminuria.17 In contrast, osteonecrosis was consistently not, or negatively,10 associated with hemolysis in the literature. Altogether, these results suggested an important role of hemolysis in the development of elevated TRV, leg ulcers, and priapism. A link between hemolysis and stroke or glomerular involvement was less unequivocal.37
From a clinical perspective, the reality of such a “hyperhemolysis” risk is questionable, because most studies that have supported the concept have suffered from biases, including the lack of reliable surrogates for hemolysis and the wide use of an unreliable marker for pulmonary hypertension (TRV or NT-proBNP level).12,13
Our study did not demonstrate any independent association between an increase of our indirect hemolysis index and the expected vascular complications when accounting for the degree of anemia. Several factors may explain this discrepancy. First, our study took place in Africa, a completely different medical, socioeconomic, and climatic context. In contrast to the African American SCD population, patients of our study did not undergo disease-modifying treatment. Less than 2% had access to iterative transfusion or hydroxycarbamide that could modify the incidence of complications as well as the level of hemolysis. Second, African patients are exposed to numerous pathogens, including malarial Plasmodia. Although patients were investigated at steady state, one cannot exclude that part of their hemolysis resulted from asymptomatic infections. However, an additional source of hemolysis should not modify the association between the hemolysis index and vascular complications if hemolysis per se is assumed to be responsible for the development of vasculopathy. Third, the genetic background may differ between the West African population and the genetically heterogeneous African American patients. In particular, genetic variants that modulate the level of HbF may have a different prevalence. HbF plays a role in both the level of hemolysis and the occurrence of SCD complications.38 In our study, HbF levels did not modify the association between vascular complications and the indirect hemolysis index (supplemental Table 4). Fourth, we found stronger associations between hemolysis markers and the vascular complications in the adult population, although their magnitude did not reach those described by Taylor et al.10 Therefore, the median age of patients in the study may influence the relationship observed between hemolysis and vasculopathy. It is conceivable that only prolonged exposure to increased hemolysis leads to endothelial damage.
Our study presents several strengths. It is the largest, and first in sub-Saharan African patients, to evaluate the association between the degree of anemia and indirect markers of hemolysis and the principal SCD-related vascular complications. Moreover, recruitment included a large range of outpatients without treatment. We were thus able to analyze the consequences of the natural history of SCD, in contrast to studies performed in high-income countries. The large sample size also allowed us to adjust the analyses for many potential confounder variables and to perform stratified analyses by age or SCD phenotype subgroups.
Our study also had several limitations. First, there may be a mortality bias, especially for stroke, because of the cross-sectional nature of the study. We only studied patients who had survived the disease thus far, thus minimizing the prevalence of lethal complications. However, the association between anemia (and hemolysis) and vascular complications may be underestimated in the surviving patients if patients with both more severe anemia (and potentially increased hemolysis) and vascular complications die earlier than other SCD patients. Noteworthy, this bias should be stronger in adults than in children, but we observed stronger associations in adults. Second, many SCD complications were clinically assessed, and some subclinical phenotypes, such as asymptomatic osteonecrosis or stroke, may have been underdiagnosed. Conversely, it is now well established that echocardiographic measurement of TRV is not a specific screening method for pulmonary hypertension, because only 25% of patients with a TRV >2.5 m/s actually have pulmonary hypertension by right heart catheterization.24 However, the previous associations between pulmonary hypertension and hyperhemolysis were established using this method, and it was neither feasible nor ethical to propose right heart catheterization to our patients. Third, we had to cope with missing laboratory data, especially for LDH levels. These data were missing for patients enrolled during the first year of inclusion. We verified that these data were missing at random, and the control of our imputation model was statistically solid (supplemental Figures 1 and 2). Moreover, the sensitivity analysis using nonimputed LDH only showed similar results, although they were less significant. Finally, we did not directly measure hemolysis, but developed an indirect hemolysis index based upon routine laboratory correlates of hemolysis that were best adapted to the available data. Likewise, none of the previous clinical studies used direct measurement of hemolysis in SCD13 because direct measurement of RBC lifespan cannot be applied on a large scale. Hemolysis is a complex process than can occur intravascularly, extravascularly, or both, and the site of hemolysis may differentially influence the levels of LDH and bilirubin. In addition, neither marker is specific for hemolysis, because they can rise in several other confounding conditions, including infectious diseases and liver disease. The reticulocyte count is correlated with both intra- and extravascular hemolysis, but mainly reflects the bone marrow response and can be imprecise whether measured by light microscopy or an automated instrument.39 Minniti et al proposed overcoming the problem of the unreliability of individual markers by combining the information of several (LDH, ASAT, total bilirubin levels, and reticulocyte percentage) and generated a “composite hemolytic component” using PCA.16 This component has never been validated against direct measurement of hemolysis. However, it was found to be highly associated with plasma RBC microparticle levels and cell-free hemoglobin concentration, which may be more direct markers of hemolysis, but are not available for routine screening. We followed the same strategy to accommodate our available data, but used LDH and bilirubin only because of the imprecision of reticulocyte counts and the high prevalence of liver diseases in Africa, which can increase ASAT levels. We also substituted a binary categorical variable of clinical icterus for bilirubin for 63% of patients, changed bilirubin from a continuous variable to a categorical variable, and imputed missing LDH values in 33% of patients. These differences may have contributed to the differences between our findings and studies using the original hemolytic component. The consistency of our indirect hemolysis index was however controlled by performing the analyses using LDH as the sole hemolysis marker as done by many authors before.10,29,31,32,35 The results were quite similar, with modest OR and less significance, because of the reduction in sample size.
In conclusion, severe anemia at steady state in SCD patients living in West and Central Africa is associated with only some specific vascular complications of the disease, including elevated TRV and microalbuminuria in the whole population and leg ulcers in SS-Sβ0 adults. These complications are not independently associated with indirect markers of increased hemolysis. It is conceivable that our indirect index does not reflect actual hemolysis, but also that other mechanisms leading to anemia, such as iron deficiency and inflammation, are involved in the pathophysiology of SCD-related vascular complications, in addition to, and independently from, hemolysis. Translational research studies are necessary to assess the interaction between SCD vasculopathy, malnutrition, and infectious diseases, particularly malaria, in sub-Saharan Africa.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was funded by the Laboratory of Excellence GrEx, Sorbonne Paris Cité University.
Authorship
Contribution: M.D. monitored the database, verified and analyzed the data, and contributed to the writing of the manuscript; J.E. contributed to the writing of the manuscript; A.T. coordinated patient recruitment and clinical explorations in Abidjan; D.A.D. coordinated patient recruitment and clinical explorations in Bamako; S.D. and I.D. coordinated patient recruitment and clinical explorations of adults and children in Dakar; I.S. coordinated patient recruitment and clinical explorations of patients in Abidjan; S.B. coordinated adult patient recruitment and biological sample collection in Yaoundé; O.G. coordinated patient recruitment and clinical explorations in Douala; G.W. coordinated pediatric patient recruitment in Yaoundé; F.N.S. coordinated adult patient recruitment in Yaoundé; K.B. and I.K. included the adults and pediatric patients in Abidjan; Y.T. and C.O.D. included the patients, monitored the clinical data, and computerized the data in Bamako; V.G. coordinated biological sample collection in Abidjan; B.F.F. and M.S. included the adult patients in Dakar; I.D.L. included the pediatric patients in Dakar; D.C. coordinated patient recruitment and cardiac explorations of patients in Yaoundé; R.N. coordinated patient recruitment and cardiac explorations of patients in Abidjan; I.B.D. coordinated patient recruitment and cardiac explorations of patients in Dakar; B.G. contributed to the statistical data; X.J. initiated the study, directed the monitoring, and oversaw the writing of the manuscript; and B.R. designed the study, coordinated patient recruitment, verified and analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brigitte Ranque, INSERM UMR_S970, Paris Descartes University, Assistance Publique Hôpitaux de Paris, 20 rue Leblanc, 75015 Paris, France; e-mail: brigitte.ranque@aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal