Abstract
Current understanding of how platelets localize coagulation to wound sites has come mainly from studies of a subpopulation of activated platelets. In this review, we summarize data from the last 4 decades that have described these platelets with a range of descriptive titles and attributes. We identify striking overlaps in the reported characteristics of these platelets, which imply a single subpopulation of versatile platelets and thus suggest that their commonality requires unification of their description. We therefore propose the term procoagulant platelet as the unifying terminology. We discuss the agonist requirements and molecular drivers for the dramatic morphological transformation platelets undergo when becoming procoagulant. Finally, we provide perspectives on the biomarker potential of procoagulant platelets for thrombotic events as well as on the possible clinical benefits of inhibitors of carbonic anhydrase enzymes and the water channel Aquaporin-1 for targeting this subpopulation of platelets as antiprocoagulant antithrombotics.
Introduction
The ability of platelets to multitask is critical to the arrest of bleeding after injury and to the development of arterial thrombosis, which underlies stroke and coronary artery disease. Following the reflex vasoconstriction of an injured vessel, platelets are recruited to the wound site to which they adhere and aggregate to form a fragile plug in the initiation of hemostasis. Platelets then provide the requisite membrane for the assembly of the prothrombinase complex, thereby localizing coagulation to the wound site. The local generation of thrombin catalyses the conversion of soluble fibrinogen to insoluble fibrin, which stabilizes the plug into a firm clot that prevents bleeding and promotes healing. It is now apparent that different populations of platelets play different roles in the cascade of events in thrombus formation.
The concept of functionally different subpopulations of platelets or division of labor among platelets at wound sites is well reported. For example, Patel et al1 described differences between “vanguard platelets,” a subpopulation that is the first to adhere to collagen, and a second population of “follower platelets” that adhere over the top of these. Furthermore, it is now well accepted that even after strong activation, not all stimulated platelets become procoagulant. Accordingly, differential expression on the plasma membrane of phosphatidylserine (PS) or components of the prothrombinase complex have been reported after platelet activation with thrombin and/or collagen2,3 (see Figure 1). Indeed, an increasing number of studies show heterogeneity in platelet responses to agonists. Two distinct phenotypes are discernible in the literature, a procoagulant phenotype that externalizes PS, binds tenase and prothrombinase complexes, and accelerates coagulation at the wound site, and an aggregating and contractile phenotype characterized by active integrin αIIbβ3, which pulls fibrin over the platelet plug to tighten the clot into an impermeable cell mass.4-7
Procoagulant attributes and causative mechanisms of candidate procoagulant platelets. Platelet phenotypes by their published names or by their unique features as follows: (1) BP/ballooned non-spread platelets (BNS)8,10,17 ; (2) SCIP17 ; (3) BAPS platelets,8,18 high-density bubble-shaped (HDBS) platelets also show similar features19 ; (4) FIB-CAP,20,21 characterized by PS exposure, the lack of active integrin αIIbβ3, and the retention of fibrinogen and thrombospondin as a single patch or cap on the procoagulant platelets; (5) 4-[N-(S-glutathionylacetyl) amino] phenylarsonic acid (GSAO) binding procoagulant platelets22,23 ; (6) procoagulant platelets dependent on the formation/opening of MPTP24-28 ; and (7) collagen and thrombin-activated (COATED) platelets.11,26-30 BP, SCIP, BAPS, and FIB-CAP platelets are broadly referred to as ballooning/BPs, whereas GSAO, MPTP, and COATED platelets are broadly classified as MPTP phenotype. The procoagulant attributes are qualitative features of procoagulant platelets, which we scored 100 for “Yes,” 0 for “No,” and 50 for transient features or “Yes/No.” Features based on the “causative mechanisms” are quantitative; for this we assigned values based on published fractional responses or the reported percentage procoagulant platelets formed. We then present these data as a color map where the upper, middle, and lower end of a 0 to 100 scale is represented by red, yellow, and blue, respectively. To determine the characteristic features of the procoagulant platelet based on pooled data, we used the mean value for each feature across candidates of the procoagulant platelet (BP, BNS, HDBS, FIB-CAP, SCIP, BAPS, GSAO, COATED, and MPTP), as shown in the corresponding row of the “PROCOAG ID” column. The color map was generated using Prism 7 (GraphPad software). White spaces within the map represent unknown parameters, whereas the black column demarcates the individual scores from the mean score in the “PROCOAG ID” column. vWF, Von Willebrand factor.
Procoagulant attributes and causative mechanisms of candidate procoagulant platelets. Platelet phenotypes by their published names or by their unique features as follows: (1) BP/ballooned non-spread platelets (BNS)8,10,17 ; (2) SCIP17 ; (3) BAPS platelets,8,18 high-density bubble-shaped (HDBS) platelets also show similar features19 ; (4) FIB-CAP,20,21 characterized by PS exposure, the lack of active integrin αIIbβ3, and the retention of fibrinogen and thrombospondin as a single patch or cap on the procoagulant platelets; (5) 4-[N-(S-glutathionylacetyl) amino] phenylarsonic acid (GSAO) binding procoagulant platelets22,23 ; (6) procoagulant platelets dependent on the formation/opening of MPTP24-28 ; and (7) collagen and thrombin-activated (COATED) platelets.11,26-30 BP, SCIP, BAPS, and FIB-CAP platelets are broadly referred to as ballooning/BPs, whereas GSAO, MPTP, and COATED platelets are broadly classified as MPTP phenotype. The procoagulant attributes are qualitative features of procoagulant platelets, which we scored 100 for “Yes,” 0 for “No,” and 50 for transient features or “Yes/No.” Features based on the “causative mechanisms” are quantitative; for this we assigned values based on published fractional responses or the reported percentage procoagulant platelets formed. We then present these data as a color map where the upper, middle, and lower end of a 0 to 100 scale is represented by red, yellow, and blue, respectively. To determine the characteristic features of the procoagulant platelet based on pooled data, we used the mean value for each feature across candidates of the procoagulant platelet (BP, BNS, HDBS, FIB-CAP, SCIP, BAPS, GSAO, COATED, and MPTP), as shown in the corresponding row of the “PROCOAG ID” column. The color map was generated using Prism 7 (GraphPad software). White spaces within the map represent unknown parameters, whereas the black column demarcates the individual scores from the mean score in the “PROCOAG ID” column. vWF, Von Willebrand factor.
Over the last 4 decades, several subpopulations of platelets with different features have been identified, named, and suggested as candidates for the procoagulant platelet. In this review, we summarize these data, reveal attributes central to these platelet phenotypes, and provide perspectives on the mechanisms and molecular targets in the procoagulant response of activated platelets. We suggest that the various phenotypes described actually form a spectrum of a single platelet phenotype, the procoagulant platelet. Finally, we examine the potential for targeting this platelet subpopulation as an antithrombotic approach. In this regard, we discuss the potential of inhibitors of the water channel aquaporin-1 (AQP1) and the repurposing of carbonic anhydrase inhibitors as antiprocoagulant antithrombotics, which may selectively limit the platelet procoagulant responses while sparing platelet aggregation and secretion.
How 1 platelet becomes procoagulant while another does not
A high and sustained calcium rise is required for PS externalization, coagulation factor binding, and calpain-mediated inactivation of αIIbβ3 integrin and is a major difference between aggregatory and procoagulant platelets, possibly explaining their different derivations.8-11 It is well known that the procoagulant response of strongly activated platelets is preceded by calcium mobilization from intracellular stores10,12,13 ; this is associated with the activation of Ca2+ activated chloride channels, resulting in an initial salt entry, which is then followed by the influx of water.8 The chloride ion entry causes membrane hyperpolarization and enhances the electrochemical drive for Ca2+ entry14 through both store-operated and store-independent pathways; together these ensure a high and sustained level of cytosolic calcium required to drive the procoagulant response.8,10,12-14 The pattern of calcium signaling is different in adherent aggregating platelets, which are rather characterized by intermittent spikes in calcium levels or oscillatory calcium responses and the sustained engagement of αIIbβ3 integrins.8,10,15,16 Furthermore, platelet age or level of oxidative stress may precommit platelets to aggregatory or procoagulant phenotypes as discussed later in this review.
Categorization of procoagulant platelet types
Platelets reported in the literature to be procoagulant show several overlapping features and may be classified as ballooning or mitochondrial permeability transition pore (MPTP) phenotypes based on the broad descriptions provided in the corresponding papers here reviewed. We depict the features of these platelets and explain their overlaps.
Balloon-shaped or ballooning phenotypes
The earliest report of a ballooning platelet phenotype was in 1978 by Wester et al, who reported the ultrastructural changes platelets undergo to arrest bleeding after skin vessel transection.31,32 Changes in platelet morphology were investigated at high resolution by electron microscopy, and a subset of platelets was observed to have assumed a ballooned shape, with fibrin deposited between platelet balloons. These ballooned platelets were an integral part of the platelet plug and stable clot.31,32 About 2 decades later, this phenotype was generated in vitro on a collagen matrix, and platelets were shown to externalize PS after a sustained cytosolic calcium rise and in the presence of physiological levels of extracellular calcium.10 Since then, several studies have investigated the procoagulant attributes of this platelet phenotype and its contribution to arterial thrombus formation.8,10,17,20,21,33,34 It is clear that ballooned platelets not only bind annexin-V as indication of PS exposure but also provide an extended surface area for the assembly of the prothrombinase complex and contribute to the acceleration of coagulation at the wound site.8,20,21,33,34 Ballooned phenotypes have been reported as either ballooned platelets (BPs),8,10,34 sustained calcium-induced platelets (SCIP),17 fibrinogen capped platelets (FIB-CAP),20,21 or ballooned and procoagulant-spread (BAPS) platelets8 (Figure 1) and show attributes similar to other candidates of the “procoagulant platelet,” such as the MPTP and COATED platelets.29,35
MPTP phenotype
In a recent study, Hua et al showed that colabeling of platelets with fluorescent conjugates of a tripeptide trivalent arsenical GSAO and an α-granule marker identified a subpopulation of activated procoagulant platelets undergoing cyclophilin D–dependent necrosis.22 GSAO likely covalently binds to proteins containing cysteine thiols36 as it has been previously reported to covalently bind the molecular chaperone heat shock protein 90.37 We have previously shown that the actin cytoskeleton undergoes remodeling and the cell membrane increases permeability during platelet transformation to the procoagulant phenotype,8 and this would allow GSAO to label intracellular proteins in necrotic platelets.22 Furthermore, GSAO platelets show striking similarities to the activated necrotic platelets described by Jobe et al24 as “highly activated platelets” characterized by high-level PS externalization, high-level fibrinogen retention, antigenic modulation of αIIbβ3, and marked membrane vesiculation. Like GSAO platelets, the formation of this subpopulation of platelets was dependent on cyclophilin D–induced MPTP formation and opening. Cyclophilin D is considered a modulatory component of MPTP, which may regulate pore opening to allow for cytosolic molecule influx, leading to increased matrix volume, disruption of mitochondrial outer membrane, and oxidative stress.38,39 Accordingly, cyclophilin D inhibition (by cyclosporin A, coenzyme Q, or bongkrekic acid) markedly reduced the formation of both MPTP and GSAO platelets in independent experiments.22,24,26 Another platelet subpopulation exhibiting the MPTP phenotype is the COATED platelet. These were originally so named by Dale et al in 2005, as a subpopulation of platelets that were generated after collagen and thrombin stimulation.27 Specific parameters characterize this PS exposing platelets, namely the surface expression of α-granule proteins such as FVa, strong binding of transglutaminase substrates, fibrinogen, von Willebrand factor, thrombospondin, fibronectin, and α2-antiplasmin.27
Commonalities of candidate procoagulant platelets
Like the ballooning phenotype of procoagulant platelets (BP, SCIP, BAPS, FIB-CAP), COATED platelets bind α-granule protein and fibrinogen,27,28 externalize PS,28 promote microvesiculation,30 and become membrane permeable after activation.28 Furthermore, convincing data also indicate COATED platelet formation is associated with rapid loss of loss of mitochondrial transmembrane potential (Δψm),26 a characteristic also described for MPTP and GSAO platelets.22,24 Other striking similarities in the formation and characteristics of the “procoagulant platelet,” as described in the literature, are shown in Figure 1. The overlap in features of these platelets is therefore strongly suggestive of a single procoagulant versatile subpopulation of platelets. We therefore propose that platelets previously described by any of these range of descriptors, that show the common characteristics indicated in the “PROCOAG-ID” column of Figure 1, be simply referred to as procoagulant platelets.
The features of the procoagulant platelet have differed depending on whether it was investigated in suspension (COATED, MPTP, BNS, FIB-CAP, or GSAO) or adherent to solid agonist-coated matrices (SCIP, MPTP, BNS, BAPS, FIB-CAP). Unlike in suspension, a solid matrix provides procoagulant platelets with an adhesion platform upon which unique morphological transformations can occur. Features such as procoagulant-spreading, observed in SCIP and BAPS platelets, have been identified after adhesion to agonist-coated surfaces,8,17 but not in suspension. The characteristics of the procoagulant platelet, identified under either adhesion or suspension conditions, are summarized in Table 1.
Features of adherent and nonadherent procoagulant platelets
| Adherent platelets . | Suspended platelets . |
|---|---|
| • PS exposing | • PS exposing |
| • FXa/ FVa binding | • FXa/FVa binding |
| • Thrombin generation | • Thrombin generation |
| • ↑ Membrane permeability | • ↑ Membrane permeability |
| • Loss of Δψm | • Loss of Δψm |
| • Mitochondrial depolarization | • Mitochondrial depolarization |
| • Cyclophilin D dependence | • Cyclophilin D dependence |
| • Microvesiculation | • Microvesiculation |
| • Ballooning | • Ballooning |
| • Procoagulant-spreading |
| Adherent platelets . | Suspended platelets . |
|---|---|
| • PS exposing | • PS exposing |
| • FXa/ FVa binding | • FXa/FVa binding |
| • Thrombin generation | • Thrombin generation |
| • ↑ Membrane permeability | • ↑ Membrane permeability |
| • Loss of Δψm | • Loss of Δψm |
| • Mitochondrial depolarization | • Mitochondrial depolarization |
| • Cyclophilin D dependence | • Cyclophilin D dependence |
| • Microvesiculation | • Microvesiculation |
| • Ballooning | • Ballooning |
| • Procoagulant-spreading |
Procoagulant platelets show similar features, in the presence of physiological concentrations of extracellular calcium, whether studied adherent to a collagen matrix or stimulated with collagen in suspension. A distinguishing feature, unique to adherent platelets, is the formation of procoagulant-spread structures. There are currently no identified features that suspended procoagulant platelets show that are unique to this mode of activation.
Mechanisms of procoagulant platelet formation
The 7 candidates for the procoagulant platelet shown in Figure 1 share a common mechanism of formation, a further indication that these comprise a spectrum of a single platelet phenotype.
Agonist requirement for procoagulant platelet formation
Collagen remains the most important single agonist for the formation of adherent procoagulant platelets in the presence of physiological concentrations of extracellular calcium; it accounts for 40% to 80% of the procoagulant platelets formed under this condition8,10 (Figure 1). In suspension, however, costimulation with agonists of protease-activated receptors was often necessary to achieve a similar size of procoagulant subpopulation.22,24 With thrombin alone, the proportion of activated platelets that become procoagulant varied from 20% to 60% in suspended platelets to 1% to 30% in platelets adhered to inert surfaces (Figure 1).These differences are likely to be due to variations in experimental conditions and to the different agonist stimulation pathways; however, in both adherent and suspended platelets, signaling via the glycoprotein VI receptor is a major pathway for the formation of procoagulant platelets. Platelet activation with adenosine diphosphate, von Willebrand factor, or a thromboxane A2 mimetic alone does not or only weakly generates procoagulant platelets.18 In addition, some studies report that platelet pretreatment with aspirin has minimal effect on the generation of procoagulant platelets.22,35
Calcium dependence of procoagulant platelets
Platelet activation via glycoprotein VI or Gq-protein–coupled protease-activated receptors leads to a sustained increase in cytosolic calcium owing to both extracellular calcium influx and calcium mobilization from stores.12,40 The role of calcium signaling in the formation of procoagulant platelets is pivotal, because formation of these platelets is abrogated by the depletion of extracellular calcium or by BAPTA-mediated chelation of cytosolic calcium8,10 (Figure 1); notably, there needs to be a sustained rise in cytosolic calcium to activate downstream signaling for a procoagulant response8,10,21,22,41 (Figure 1).
The role of calpain
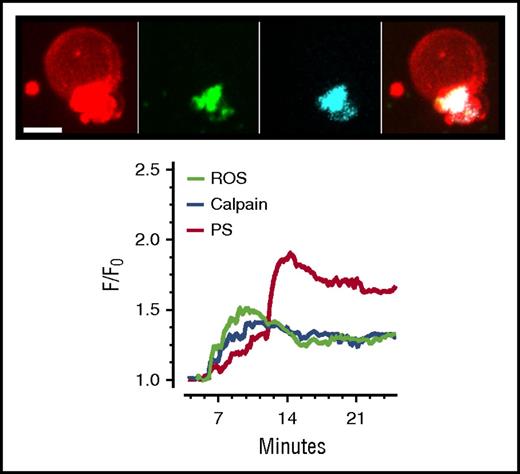
Sustained elevated cytosolic calcium required for the procoagulant response will in tandem breach the threshold of [Ca2+]Cyt required to activate the thiol protease, calpain.17,42 Major contractile cytoskeletal and membrane linker proteins, such as actin, vinculin, and myosin, have been identified as calpain substrates.43 Once activated, calpain may induce the proteolysis of these proteins and aid the remodeling associated with the dramatic transformation of the procoagulant platelet. The fragmentation of the procoagulant platelet membrane and eventual release of PS-positive microvesicles are consistent with calpain action.44 Indeed, we recorded a rise in levels of activated calpain during active membrane ballooning in collagen-activated platelets (Figure 2), consistent with previous reports of calpain activation in collagen-stimulated platelets.45,46
Spatiotemporal pattern of calpain activation, PS exposure, and reactive oxygen species generation in the ballooning platelet. Extended focus images obtained at 20 minutes after platelet adherence to fibrillar collagen show the spatial location of exposed PS indicated in red (as monitored by membrane annexin-V accumulation), calpain indicated in cyan, and reactive oxygen species (ROS) indicated in green. Calpain activity was monitored by the 7-amino-4-chloromethylcoumarin (CMAC)-based substrate, fluorogenic t-BOC-Leu-Met-CMAC substrate, which yields fluorescent peptidase products with improved retention in live platelets. The generation of ROS during membrane ballooning was followed in real time by means of MitoSox (ThermoFisher Scientific), which is rapidly oxidized by superoxide to produce highly fluorescent products. The chart shows the temporal profile of calpain activation, PS exposure, and ROS generation in a ballooning human platelet. Written informed consent was obtained in accordance with the Declaration of Helsinki. Human blood was obtained from healthy, drug-free volunteers under the University of Bristol, United Kingdom, Research Ethics approval (E5736). Live cell imaging was performed at 25°C using a spinning-disk confocal system as previously described.8,18 Scale bar represents 3 µm. Data are representative of 4 independent experiments. F/F0, relative fluorescence intensity over time, where F0 is the background-subtracted fluorescence intensity before platelet activation.
Spatiotemporal pattern of calpain activation, PS exposure, and reactive oxygen species generation in the ballooning platelet. Extended focus images obtained at 20 minutes after platelet adherence to fibrillar collagen show the spatial location of exposed PS indicated in red (as monitored by membrane annexin-V accumulation), calpain indicated in cyan, and reactive oxygen species (ROS) indicated in green. Calpain activity was monitored by the 7-amino-4-chloromethylcoumarin (CMAC)-based substrate, fluorogenic t-BOC-Leu-Met-CMAC substrate, which yields fluorescent peptidase products with improved retention in live platelets. The generation of ROS during membrane ballooning was followed in real time by means of MitoSox (ThermoFisher Scientific), which is rapidly oxidized by superoxide to produce highly fluorescent products. The chart shows the temporal profile of calpain activation, PS exposure, and ROS generation in a ballooning human platelet. Written informed consent was obtained in accordance with the Declaration of Helsinki. Human blood was obtained from healthy, drug-free volunteers under the University of Bristol, United Kingdom, Research Ethics approval (E5736). Live cell imaging was performed at 25°C using a spinning-disk confocal system as previously described.8,18 Scale bar represents 3 µm. Data are representative of 4 independent experiments. F/F0, relative fluorescence intensity over time, where F0 is the background-subtracted fluorescence intensity before platelet activation.
Integrin activation
The structure and function of the platelet integrin αIIbβ3 are extensively reviewed elsewhere.47,48 This integrin is the best characterized of the platelet integrins, yet reports of its role in the formation of procoagulant platelets are conflicting.8,21,25 For example, after collagen stimulation, the BAPS phenotype of platelets showed high fibrinogen binding and αIIbβ3 activation; however, BP, COATED, SCIP, MPTP, and FIB-CAP platelets vary in this respect9 (Figure 1). Some of the discrepancies may result from differences in kinetics of integrin activation. Platelets stimulated with convulxin/thrombin showed high fibrin and fibrinogen binding, which was associated with decreased PAC-1 binding after an initial peak11 ; this supports previous reports of secondary inactivation of integrin αIIbβ39,17 and a conclusion that its activity during the formation of procoagulant platelets is transient. Moreover, it is possible that fibrinogen and fibrin bind to distinct sites on integrin αIIbβ3,15,49-51 and binding to each may be modulated differently by the mechanisms of integrin inactivation.
Chloride ion (Cl−) entry and a role for TMEM16F
Our pooled data indicate an association between the platelet procoagulant response and Cl− entry (Figure 1); strong stimulation of platelets with collagen and or thrombin induce Cl− entry, which drives membrane hyperpolarization and PS exposure.14 There is also good evidence that TMEM16F or Ano6 is a key channel for Cl− entry in this response.52,53 Indeed, ablation of TMEM16F54 or the blockade of calcium-activated Cl− channels with small molecule inhibitors markedly diminished the platelet procoagulant response.8 Accordingly, membrane ballooning was ablated in the Scott patient’s platelets, thus indicating an important role for TMEM16F in the platelet procoagulant response.8,9,53-57
The procoagulant platelet is under oxidative stress
Independent of granular release, the oxidizing agent H2O2 alone can induce a cyclophilin D–dependent loss of mitochondrial transmembrane potential (Δψm) due to MPTP formation and opening24 ; this effect is calcium independent and is mediated by thiol oxidation.58,59 The pooled evidence (Figure 1) indicates that thrombin alone is a weak agonist for inducing the procoagulant response in activated platelets; however, once under oxidative stress, platelets are 6 times more likely to become procoagulant with thrombin stimulation alone.24 This suggests a role for intracellular oxidative stress and reveals an ancillary pathway independent of strong stimulation for the formation of the “procoagulant platelet.” Interestingly, oxidative stress alone has been shown to have no effect on fibrinogen binding, PS externalization, or granule release, raising the possibility that intravascular oxidative stress may play a role only in the priming of platelets for procoagulant response. This fits the observation that platelet aging, which has been reported to be associated with glutathione peroxidase-1 or nicotinamide adenine dinucleotide phosphate oxidase dysfunction and oxidative stress,60,61 may also prime and commit platelets to the procoagulant pathway. Equally, drugs or oxidizing agents that potentiated platelet oxidative stress or MPTP formation/opening have been shown to promote the formation of procoagulant platelets.24,26 Consistent with these and related findings previously reviewed,60 we observed that the procoagulant response of human platelets adhering to collagen was associated with increased superoxide generation (Figure 2).
A phenotype that gains function by dying
Although it is clear that procoagulant platelets are undergoing a death process, the literature is ambivalent on whether this is by apoptosis26,62 or necrosis,8,22 indicating that the process may be strictly neither. Instead, it follows a pathway analogous to necrosis, but differentiated by a gain of procoagulant function. This pathway is induced by external ligand and characterized by the following features: (1) calcium dependence,8,10,12,18 (2) cyclophilin D but not Bax/Bak ablation dependence,22,24 (3) formation/opening of MPTP,24,58 (4) early and rapid loss of Δψm,24 (5) intracellular oxidative stress,8,22,26 (6) membrane ballooning due to a tightly regulated fluid entry system,8,10 (7) an early increase in membrane permeability associated with microtubule unwinding, and (8) calpain-mediated remodeling of the actin-cytoskeleton.44,63 Characteristically, these events are caspase independent (Figure 1), yet they drive the amplification of thrombin generation through the combined increase in membrane PS exposure and membrane surface area and microvesiculation.8,18,24 It is interesting to note that although cell death is classically associated with a loss of function, for the procoagulant platelet, the death process is clearly mechanistically important.
The procoagulant response is regulated by fluid entry
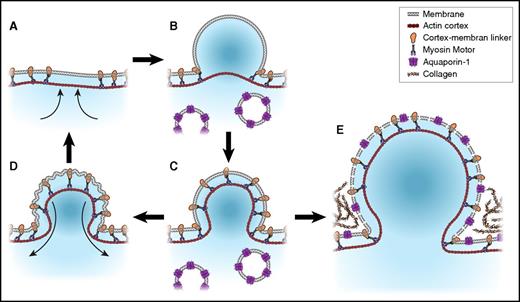
A key event during procoagulant platelet formation is the irreversible membrane swelling or ballooning that results from the physical disruption of the membrane-cytoskeleton interaction, and an increase in internal hydrostatic pressure provided by a coordinated fluid entry system.8 Balloon formation is reported in adherent platelets8,18 as well as in stimulated platelet suspensions9,64 ; in Figure 3, we illustrate its formation mechanism. The fluid entry requirement for ballooning distinguishes this process from blebbing, which is the formation of retractable membrane protrusions. Blebbing is often used interchangeably with “ballooning,”10,56 whereas both events illustrated in Figure 3 are distinct and driven by separate mechanisms.8 For example, (1) balloons, but not transient membrane blebs, are procoagulant; (2) ballooning but not bleb formation requires disruption to the platelet microtubule cytoskeleton and increased membrane permeability; (3) unlike blebs, procoagulant ballooning is irreversible and consequent upon Na+, Cl−, and water entry; and (4) whereas the hydrostatic pressure required for bleb formation is fluid entry independent, the rapid membrane expansion associated with ballooning requires fluid entry driven by the osmotic pressure of salt entry8 (Figure 3). Precision is therefore required when discussing these events in platelets.
Membrane blebbing and ballooning in human platelets. Membrane blebbing is initiated by localized cortical actin contraction (A),65 which weakens the membrane-cytoskeleton interaction and allows internal hydrostatic pressure to drive membrane protrusions (<1 µm diameter). This may be accompanied by a further detachment of the membrane from the cortex and a total volume change of <10% (B). The recruitment of myosin to the expanded cortex enables bleb retraction (C-D).65,66 Blebs were shown to form at 1 or more sites on platelet membranes upon contact with collagen and are retractable and may be re-formed.8 At some point, usually a single bleb would undergo a rapid increase in volume, resulting in a characteristic platelet balloon (E).8
Membrane blebbing and ballooning in human platelets. Membrane blebbing is initiated by localized cortical actin contraction (A),65 which weakens the membrane-cytoskeleton interaction and allows internal hydrostatic pressure to drive membrane protrusions (<1 µm diameter). This may be accompanied by a further detachment of the membrane from the cortex and a total volume change of <10% (B). The recruitment of myosin to the expanded cortex enables bleb retraction (C-D).65,66 Blebs were shown to form at 1 or more sites on platelet membranes upon contact with collagen and are retractable and may be re-formed.8 At some point, usually a single bleb would undergo a rapid increase in volume, resulting in a characteristic platelet balloon (E).8
Clinical relevance and translational potential for procoagulant platelets
Globally, cardiovascular disorders (CVDs) remain the largest single contributor to mortality and morbidity.67,68 The role of the procoagulant platelet in platelet-driven thrombosis may underlie this statistic. For example, on the 1 hand, low levels of procoagulant platelets have been shown to correlate with increased frequency of acute ischemic stroke complications, especially after thrombolytic therapy and increased spontaneous intracranial hemorrhage69-71 ; on the other hand, high levels of these platelets correlate with transient ischemic attack and stroke72,73 and with milder hemorrhagic phenotype in severe hemophilia.74 It may therefore be possible to exploit procoagulant platelets either through targeting them pharmacologically or through identifying them as biomarkers, in the management of thrombotic cardiovascular disease.
Procoagulant platelets as clinical biomarkers
There is a need for universally accepted markers and/or identity criteria for the clinical assessment of thrombotic or bleeding tendency. It is possible that clinical markers could be designed based on the shared attributes of procoagulant platelets, and their identity criteria could be based on their commonalities shown in Figure 1. For example, evaluation of blood from healthy donors for procoagulant platelets, based on the combined expression of activated coagulation proteins and PS externalization, showed that ∼31.67% ± 13.2% (mean ± standard deviation) of platelets can assume this phenotype after dual stimulation with collagen and thrombin, as previously reported.27,72 This figure varied widely between donors but appeared conserved within donors over time,29 a clinical feature that may be exploited to individualize and monitor bleeding or thrombotic tendency in anticoagulation therapy or surgical units. Furthermore, classical features of the procoagulant platelet, such as increased membrane permeability,8 will enable their in vitro identification by quick tests utilizing low-molecular-weight cell-impermeant dyes such as propidium iodide, which label DNA and RNA.75 Also, the report of GSAO earlier described as a marker of procoagulant platelets demonstrates the feasibility of this approach.22 Other features, such as a strong fibrinogen or coagulation factors (FVa, FXa) binding (Figure 1), can also be used to identify and quantify the preponderance of procoagulant platelets in clinical settings, using image-based or flow cytometric analysis.3 Such assessment, when done before major surgical intervention, may provide reliable data to enable the prediction of whether a patient has a tendency to bleed or will be at risk of thrombosis. In addition, these data provide a lead for the development of new bedside medical devices, for measuring platelet function and with predictive capabilities for bleeding disorders. For example, the in vitro formation of BP, BAPS, FIB-CAP, GSAO, or COATED platelets was a strong correlate of in vivo arterial thrombus formation (Figure 1).
Molecular drivers of procoagulant membrane dynamics as new antithrombotic targets
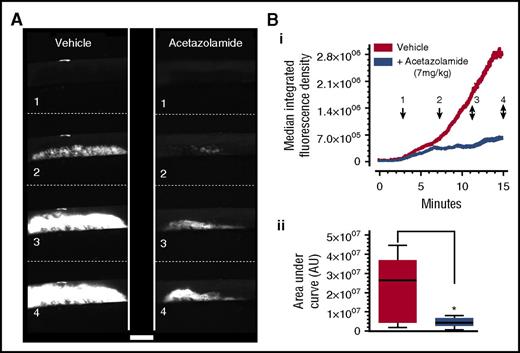
Newer data provide insight into the molecular mediators and drivers of the platelet procoagulant response.8,18,34 These molecular mechanisms reveal alternative targets for the regulation of thrombosis, which may spare essential secretion and other platelet functions.76,77 Because we had recently shown that blockade of P2Y1 and P2Y12 does not inhibit platelet procoagulant balloon formation or microvesiculation,18 there is an opportunity for development of a new class of antithrombotics that target platelet procoagulation. For example, carbonic anhydrases 1, 2, and 13 have been recently identified as new potential antithrombotic targets,77,78 for which there are already inhibitors in clinical use, such as the mild diuretic acetazolamide. We have recently shown that acetazolamide is a potent antithrombotic in vivo (Figure 4 and Agbani et al8,77,78 ) and has the potential to be a genuinely novel approach to the management of thrombotic disease. The signaling pathway involved in the antithrombotic actions of acetazolamide might include the regulation of reactive oxygen species by carbonic anhydrase enzymes, but at the moment there is no evidence in the literature. However, several reports indicate that carbonic anhydrase inhibitors are also capable of blocking water entry via the water channel AQP1,79-82 and this may also be an important mechanism of action. Consistent with this, we have recently shown AQP1 to potently regulate thrombus formation in vivo,76 implicating it as a target for development of novel antithrombotic drugs.83,84
Acetazolamide suppresses thrombus formation in vivo. Mice were administered acetazolamide (7 mg/kg) or vehicle by single bolus intravenous injection, followed immediately by DyLight 488–conjugated anti-GPIbβ antibody to label platelets. Carotid artery damage was achieved by treatment with FeCl3 as previously described.8 Fluorescently labeled platelets adhering at the site of injury could then be imaged continuously by intravital fluorescence microscopy. Images at frames indicated in panel A correspond to time points indicated in panel Bi, which shows median fluorescence intensity, quantified by using ImageJ. Analysis of the area under the curve for media fluorescence is shown in panel Bii as interleaved box plots with whiskers showing minimum to maximum values, median, and interquartile range. Data analysis was by Wilcoxon signed rank test, P < .05. *Considered significant. Scale bar, 500 µm. Data are from 8 pairs of mice. Reproduced with permission from Agbani et al.8
Acetazolamide suppresses thrombus formation in vivo. Mice were administered acetazolamide (7 mg/kg) or vehicle by single bolus intravenous injection, followed immediately by DyLight 488–conjugated anti-GPIbβ antibody to label platelets. Carotid artery damage was achieved by treatment with FeCl3 as previously described.8 Fluorescently labeled platelets adhering at the site of injury could then be imaged continuously by intravital fluorescence microscopy. Images at frames indicated in panel A correspond to time points indicated in panel Bi, which shows median fluorescence intensity, quantified by using ImageJ. Analysis of the area under the curve for media fluorescence is shown in panel Bii as interleaved box plots with whiskers showing minimum to maximum values, median, and interquartile range. Data analysis was by Wilcoxon signed rank test, P < .05. *Considered significant. Scale bar, 500 µm. Data are from 8 pairs of mice. Reproduced with permission from Agbani et al.8
A major clinical side effect associated with current antithrombotic regimens in the management of CVDs is the significant bleeding resulting from the use of dual antiplatelet therapy, for example, aspirin and P2Y12 blockers usually targeting the inhibition of platelet secretion. This status quo therefore demonstrates a need for alternative targets for the control of thrombotic events associated with CVDs. With the procoagulant response of activated platelets critically reliant on morphological transformations, molecular mediators critical to the fluid entry mechanism that drives this event provide new approaches or target genes for the control of bleeding and thrombotic disorders. Furthermore, this may reveal new molecular mechanisms in diseases associated with disorders of hemostasis. It is well established that platelets secrete a plethora of releasates essential for the maintenance of vascular integrity and cardiovascular hemostasis.85 Consequently, a new antithrombotic approach that spares essential platelet secretion will potentially limit or eliminate the well-known side effects of antiplatelet therapies.
It is rather surprising that 25% or more of patients on antiplatelet drugs go on to suffer an ischemic event.86 Controlled trials of aspirin for the long-term secondary prevention of ischemic events reports only a 13% relative reduction in risk of recurrent stroke.87,88 Similar low percent reductions were reported in studies evaluating the effects of aspirin in the 4-week risk period of recurrent stroke or intracerebral hemorrhage after short-term treatment of stroke. Together, these indicate that the present “secretion-driven” approach to controlling platelet function in thrombosis is not optimal. Also, newer data show that platelets pretreated with aspirin or from patients administered aspirin still showed full procoagulant response upon stimulation22,89 (Figure 1). The heterogeneity of activated platelets comes with unique challenges for drug therapy; aspirin, for instance, will likely affect only aggregating platelets, which may present as conventional spread, nonballooning platelets on collagen matrix.8 Therefore, coadministration of agents like acetazolamide that suppress platelet procoagulant responses and thrombus formation by distinct mechanisms may prove effective to limit the procoagulant response of platelets in patients already on aspirin. The existing literature suggests a need for such clinically effective antiplatelet-antiprocoagulant regimen.86
Potential challenges of procoagulant platelet inhibition
Platelet procoagulant activity has been linked to stroke and coronary artery disease70,90,91 ; however, this activity is also vital for hemostasis after vessel injury.70 Thus, its blockade as an antithrombotic approach may precipitate a bleeding diathesis if not fine tuned. For example, the bleeding defect of Scott patients may be attributable to an aberrant procoagulant activity.8,92,93 Also, prolonged bleeding times have been reported in TMEM16F null mice, which showed a deficiency in Ca2+-dependent PS exposure and procoagulant activity in platelets.53,55 Fine tuning of therapy may therefore be required. Targeting platelet aquaporins, however, may achieve this fine tuning, because we have recently shown that mice lacking AQP1 show normal hemostasis, whereas thrombus formation was significantly attenuated.76 In addition, it may be possible to envisage the use of lower-dose acetazolamide in combination with lower-dose aspirin, thereby achieving antithrombotic activity at doses lower than currently recognized to cause their clinical adverse effects.
Conclusion
The major platelet phenotypes identified over the last 40 years to support coagulation are essentially a versatile subpopulation of activated platelets, which we now suggest be referred to as procoagulant platelets. Procoagulant platelets undergo morphological transformations, which amplify the surface area required for PS exposure and the acceleration of coagulation. Molecular drivers of procoagulant membrane dynamics provide distinct targets for the regulation of procoagulant platelets’ role in thrombosis. Targeting the water channel AQP1 or carbonic anhydrase enzymes may therefore represent novel approaches in the management of thrombotic disease, both arterial and venous, with potential to suppress the function of procoagulant platelets and selectively limit thrombosis with minimal effect on other platelet functions.
Acknowledgments
The authors acknowledge the Medical Research Council and the Wolfson Foundation for funding the University of Bristol Bioimaging Facility. This work was supported by a British Heart Foundation programme grant (RG/15/16/31758) (A.W.P.), the NIHR Bristol Biomedical Research Centre, and a British Heart Foundation project grant (PG/16/102/32647) (A.W.P. and E.O.A.).
Authorship
Contribution: E.O.A. and A.W.P. wrote the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ejaife O. Agbani, School of Physiology, Pharmacology, and Neuroscience, University of Bristol, Biomedical Sciences Building, Bristol, BS8 1TD, United Kingdom; e-mail: e.agbani@bristol.ac.uk; and Alastair W. Poole, School of Physiology, Pharmacology, and Neuroscience, University of Bristol, Biomedical Sciences Building, Bristol, BS8 1TD, United Kingdom; e-mail: a.poole@bris.ac.uk.

![Figure 1. Procoagulant attributes and causative mechanisms of candidate procoagulant platelets. Platelet phenotypes by their published names or by their unique features as follows: (1) BP/ballooned non-spread platelets (BNS)8,10,17; (2) SCIP17; (3) BAPS platelets,8,18 high-density bubble-shaped (HDBS) platelets also show similar features19; (4) FIB-CAP,20,21 characterized by PS exposure, the lack of active integrin αIIbβ3, and the retention of fibrinogen and thrombospondin as a single patch or cap on the procoagulant platelets; (5) 4-[N-(S-glutathionylacetyl) amino] phenylarsonic acid (GSAO) binding procoagulant platelets22,23; (6) procoagulant platelets dependent on the formation/opening of MPTP24-28; and (7) collagen and thrombin-activated (COATED) platelets.11,26-30 BP, SCIP, BAPS, and FIB-CAP platelets are broadly referred to as ballooning/BPs, whereas GSAO, MPTP, and COATED platelets are broadly classified as MPTP phenotype. The procoagulant attributes are qualitative features of procoagulant platelets, which we scored 100 for “Yes,” 0 for “No,” and 50 for transient features or “Yes/No.” Features based on the “causative mechanisms” are quantitative; for this we assigned values based on published fractional responses or the reported percentage procoagulant platelets formed. We then present these data as a color map where the upper, middle, and lower end of a 0 to 100 scale is represented by red, yellow, and blue, respectively. To determine the characteristic features of the procoagulant platelet based on pooled data, we used the mean value for each feature across candidates of the procoagulant platelet (BP, BNS, HDBS, FIB-CAP, SCIP, BAPS, GSAO, COATED, and MPTP), as shown in the corresponding row of the “PROCOAG ID” column. The color map was generated using Prism 7 (GraphPad software). White spaces within the map represent unknown parameters, whereas the black column demarcates the individual scores from the mean score in the “PROCOAG ID” column. vWF, Von Willebrand factor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/20/10.1182_blood-2017-05-787259/4/m_blood787259f1.jpeg?Expires=1769098833&Signature=uTGPjCg06bj1ic4exz7oFTYUCJR5lt9UNDh0zd3lFeP9sGh35oI67AKVEAAIWZib4tCsRpsPnLQyYx85XmYmnjv0JfvKRgHIzAN6YGFb3fYXA0itl9wnkchulMiYaZ44wwDq2bPYCgBR3eYdw8XkFXZwP4wxpnBmxJHylA8Nt0Kl0D5kITrlCJmCHZ6aelp7-yD7m8NjpJqetEMpDnIfoP-vWki1KE~cxzsLLpDDZLutMvGd4Y2V4ubFVK3YKMQF-rtp8TJYN0THDQlW8kEAuAxkS7Kenp3zinGUaxTwMzGzZoRCRrjJiMxsvVP8RWInYo3hBmnci1ODro4FoLZXew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)