In this issue of Blood, Colucci et al add to the understanding of hepcidin regulation by demonstrating a role for the immunophilin FKBP12 in dampening signaling via the bone morphogenetic protein (BMP) type I receptor ALK2.1
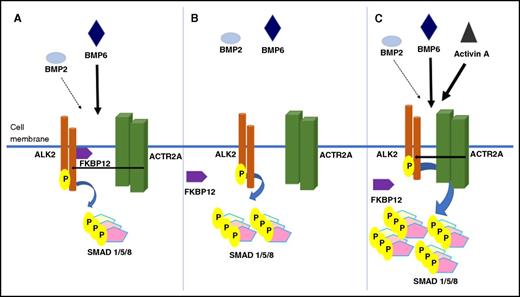
FKBP12 as a regulator of BMP/SMAD signaling. (A) When bound to ALK2, FKBP12 limits phosphorylation of SMAD 1/5/8 in response to canonical BMP signaling ligands. Upon release of FKBP12, which can occur either by mutation of ALK2 or by drug-mediated sequestration of FKBP12, ALK2 demonstrates enhanced signaling that is independent of ligand binding and phosphorylation by BMP type II receptors (B) and hyperresponsiveness to canonical BMP ligands and acquired responsiveness to the inflammatory ligand, activin A (C).
FKBP12 as a regulator of BMP/SMAD signaling. (A) When bound to ALK2, FKBP12 limits phosphorylation of SMAD 1/5/8 in response to canonical BMP signaling ligands. Upon release of FKBP12, which can occur either by mutation of ALK2 or by drug-mediated sequestration of FKBP12, ALK2 demonstrates enhanced signaling that is independent of ligand binding and phosphorylation by BMP type II receptors (B) and hyperresponsiveness to canonical BMP ligands and acquired responsiveness to the inflammatory ligand, activin A (C).
Hepatocellular expression of the iron-regulatory hormone hepcidin is influenced by several stimuli, including circulating iron levels, erythropoietic activity, and inflammation. The response to iron is mediated by BMP/SMAD signaling; moreover, a complete response to inflammation requires an intact BMP/SMAD pathway. Recent studies using genome-wide RNA interference knockdown technology identified the nutrient-sensing mammalian target of rapamycin (mTOR) pathway as a modifier of hepcidin expression.2 Rapamycin was found to increase hepcidin expression in cell culture. Hepcidin-mediated iron restriction might explain the microcytic anemia seen in association with pharmaceutical use of rapamycin.3 Colucci et al dissect the mechanism and demonstrate that rapamycin and the related drug, tacrolimus, upregulate hepcidin by sequestering the FK506 (tacrolimus) binding protein, FKBP12, to upregulate BMP/SMAD signaling. They provide evidence that this immunophilin otherwise serves as a “brake” on hepcidin signaling by its interaction with BMP type I receptor ALK2 and demonstrate increased basal hepcidin expression in cell culture systems by drug-mediated sequestration of FKBP12 or by mutation of ALK2 protein residues required for FKBP12-ALK2 interaction (see figure).
The consequences of mutations in ALK2 that interfere with FKBP12 binding on BMP/SMAD signaling have been previously investigated, because such mutations are responsible for the rare bone disorder fibrodysplasia ossificans progressiva (FOP). Empirical observations and molecular modeling suggest that causative ALK2 mutations result in a gain of function characterized by increased BMP/SMAD signaling (even in the absence of ligand binding and BMP type II receptor phosphorylation). ALK2 also shows enhanced responsiveness to canonical BMP ligands, with 1 study reporting hyperresponsiveness to BMP2 but an unaltered response to BMP6 in a hepatic cell line.4 Importantly, these mutations render the ALK2 receptor responsive to the transforming growth factor β signaling superfamily cytokine activin A. Activin responsiveness is required to recapitulate the disease phenotype in a mouse model of FOP, including the heterotopic ossification that characterizes the condition.4
Colucci et al likewise found that the consequences of abrogating FKBP12-ALK2 interactions on hepcidin expression are much more pronounced when examined in the presence of activin A. The authors suggest that during inflammation, ALK2 is not bound to FKBP12 and is thus rendered sensitive to noncanonical ligands such as activin A. This observation is particularly important, given evidence that ALK2 appears to contribute to the upregulation of hepcidin under inflammatory conditions. This model has implications for iron homeostasis during inflammation, as was suggested by a recent study reporting that the drug momelotinib decreases hepcidin production in a rat model of anemia of chronic disease by inhibiting ALK2.5 Studies using follistatin (an activin A and B inhibitor) in murine models of acute and chronic inflammation have suggested a role for one or both of these cytokines in hepcidin regulation.6 Subsequent studies have not supported a role for activin B.7
Although these studies suggest participation of ALK2 and activin A in the upregulation of hepcidin during inflammation, some lines of evidence suggest that neither is essential. For example, interleukin-6 administration induces hepcidin expression and decreases serum iron in mice with hepatocyte-specific deficiency of ALK2.8 Mice with hepatocellular inactivation of activin B do not demonstrate upregulation of SMAD5 yet have complete induction of hepcidin expression in response to endotoxin challenge or E. coli infection, suggesting that SMAD phosphorylation (or at least SMAD5 phosphorylation) might be dispensable for this response.7 Lipopolysaccharide challenge in mice was found to decrease rather than increase the transcription of the gene-encoding activin A.9
Not all of the effects of rapamycin on iron metabolism are attributable to its interaction with FKBP12 or direct effects on hepcidin expression. mTOR also modulates expression of tristetraprolin to influence transferrin receptor 1 stability and modulates phosphorylation of the iron-sulfur cluster assembly enzyme.10 Nonetheless, this study identifies FKBP12 as a novel molecular participant in the regulation of iron metabolism and, with due consideration of the lack of specificity, a potential pharmaceutical target.
Conflict-of-interest disclosure: R.E.F. is a member of the scientific advisory board of Protagonist Therapeutics. N.L.P. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal