Key Points
Vps34 controls intracellular trafficking, migration, and platelet production in MKs.
Vps34 and its stimulation-dependent PI3P production regulate platelet secretion and thrombus growth.
Abstract
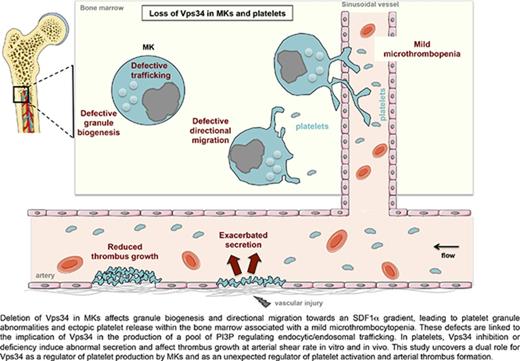
To uncover the role of Vps34, the sole class III phosphoinositide 3-kinase (PI3K), in megakaryocytes (MKs) and platelets, we created a mouse model with Vps34 deletion in the MK/platelet lineage (Pf4-Cre/Vps34lox/lox). Deletion of Vps34 in MKs led to the loss of its regulator protein, Vps15, and was associated with microthrombocytopenia and platelet granule abnormalities. Although Vps34 deficiency did not affect MK polyploidisation or proplatelet formation, it dampened MK granule biogenesis and directional migration toward an SDF1α gradient, leading to ectopic platelet release within the bone marrow. In MKs, the level of phosphatidylinositol 3-monophosphate (PI3P) was significantly reduced by Vps34 deletion, resulting in endocytic/trafficking defects. In platelets, the basal level of PI3P was only slightly affected by Vps34 loss, whereas the stimulation-dependent pool of PI3P was significantly decreased. Accordingly, a significant increase in the specific activity of Vps34 lipid kinase was observed after acute platelet stimulation. Similar to Vps34-deficient platelets, ex vivo treatment of wild-type mouse or human platelets with the Vps34-specific inhibitors, SAR405 and VPS34-IN1, induced abnormal secretion and affected thrombus growth at arterial shear rate, indicating a role for Vps34 kinase activity in platelet activation, independent from its role in MKs. In vivo, Vps34 deficiency had no impact on tail bleeding time, but significantly reduced platelet prothrombotic capacity after carotid injury. This study uncovers a dual role for Vps34 as a regulator of platelet production by MKs and as an unexpected regulator of platelet activation and arterial thrombus formation dynamics.
Introduction
Phosphoinositide 3-kinases (PI3Ks) are important lipid kinases that produce D3-phosphorylated phosphoinositides that organize functional protein complexes regulating various biological processes.1,2 The sole class III PI3K, Vps34, represents the most ancient form of PI3Ks and was initially identified in yeast with an essential role in vacuolar homeostasis.3 Vps34 occurs in complex with its regulatory protein kinase subunit, Vps15, and specifically catalyzes the conversion of phosphatidylinositol into phosphatidylinositol 3-monophosphate (PI3P). This lipid, present in relatively low amounts in cells, is known to play a central role in the regulation of intracellular trafficking.4 In mammals, Vps34 has been shown to regulate endosomal trafficking, autophagosome formation, and mTOR activation through PI3P production.5 Recently, the organismal role of Vps34 has been investigated by using mouse gene targeting strategies. Loss of the Pik3c3 gene in mice causes early embryonic lethality (between embryonic day 7.5 and 8.5) due to severe decreased cell proliferation capacity.6 In vivo studies using the tissue-specific cre-lox system have shown an important role for Vps34 in heart,7,8 liver,7 T-cell,9 podocyte,10,11 and skeletal muscle12 functions and in sensory, cortical, and hippocampal neuron integrity13,14 by regulating, through 2 different protein complexes, the endosomal and autophagic pathways.
At present, the role of Vps34 and its lipid product, PI3P, remains unknown in megakaryocytes (MKs) and platelets. Platelets are small anucleated blood cells that play a pivotal role in preventing excessive blood loss in response to vascular injury by adhering to the exposed extracellular matrix and forming a hemostatic plug. Platelets are also strongly involved in atherothrombosis, a major cause of death worldwide, and are the target of antithrombotic drugs. To produce ∼1011 platelets into the circulation daily, MKs migrate close to bone marrow sinusoids and protrude long cytoplasmic extensions called proplatelets into the sinusoidal lumen to release de novo circulating platelets.15 In MKs and platelets, endocytosis and intracellular trafficking are involved in secretory granule biogenesis as well as receptor and organelle trafficking.16-19
The aim of this study was to define the role for the Vps34/PI3P axis in MK differentiation and platelet production and function. This is important to provide new insights into these complex mechanisms and because Vps34 inhibitors, which may affect hemostasis, are under development to improve chemotherapy.20,21 To tackle this, we used both a genetic approach in mice and pharmacological inhibitors. We show that Vps34 has 2 important roles: (1) in platelet production in MKs, where it is involved in the production of an important pool of PI3P, controls MK migration and the subsequent platelet release in the blood, and regulates trafficking and granule biogenesis; and (2) in platelet functions, where it controls the production of a stimulation-dependent pool of PI3P that modulates platelet secretion and thrombus growth under arterial shear stress. This study provides new insights into the importance of the Vps34/PI3P axis as a regulator of both platelet production by MKs and platelet activation during arterial thrombus growth.
Methods
Animals
Eight- to 14-wk-old C57BL/6 mice were used for all experiments and housed in the Anexplo vivarium (US006/Centre régional d'exploration fonctionnelle et de ressources expérimentales [CREFRE], Inserm/Université Paul Sabatier, Toulouse, France) according to institutional guidelines. Ethical approval for animal experiments was obtained from the French Ministry of Research in agreement with European Union guidelines.
Human blood samples
Heparinized blood from healthy donors was purchased from the Etablissement Français du Sang (Toulouse, France) and immediately processed for experiments.
In vitro PI3K activity assay and lipid analysis
Supplemental data
Primary MK purification and culture, immunofluorescence, and flow cytometry on mature MKs as well as electron microscopy on native bone marrow and proplatelet formation assay from bone marrow explants are described in the supplemental Methods (available on the Blood Web site).
MK migration assay
Chemotaxis was assessed by using a Dunn chamber (Chemotaxis Dunn, Hawksley). MKs were allowed to adhere onto fibronectin-coated coverslips (20 μg/mL) at 37°C for 1 hour. The coverslips were then placed into the Dunn chamber where the inner well was filled with serum-free medium, and the outer well was filled with serum-free medium containing 300 ng/mL of SDF1α. Videomicroscopy was performed for 6 hours with an Axio Observer.Z1 inverted microscope operated with Zen software (Carl Zeiss) by using an ORCA R2 camera (Hamamatsu, Japan) and a 10×, 0.30 EC Plan Neofluar objective lens. Migration analysis was performed by using ImageJ software (National Institutes of Health) and the Chemotaxis Tool plug-in.
Bone marrow immunostaining
Mouse femora were fixed with 4% paraformaldehyde and 5 mM sucrose and dehydrated by using a graded sucrose series. Subsequently, the samples were embedded in OCT matrix (CellPath) and shock-frozen in liquid nitrogen. Five-micrometer–thick cryosections were probed with rabbit anti-human von Willebrand factor (vWF) antibody to specifically label MKs and platelets and mouse/rat anti-mouse FABP4/A-FABP antibody to stain microvascular endothelial cells,25 followed by Alexa Fluor secondary antibodies. Nuclei were stained by using 4′,6-diamidino-2-phenylindole. Samples were visualized with an LSM780 confocal microscope operated with Zen software (Carl Zeiss) using a 63×, 1.4 numerical aperture Plan-Apochromatic objective lens (Carl Zeiss). Image analysis was performed by using ImageJ software.
Supplemental data
Platelet life span, immune-induced thrombocytopenia, carotid artery thrombosis, and tail bleeding time are described in the supplemental Methods.
Washed murine platelet preparation, flow cytometry, electron microscopy, aggregation, secretion, αIIbβ3 integrin function assays and thrombus formation assays on collagen are described in the supplemental Methods.
Statistical analysis
Data are expressed as means ± standard error of the means (SEMs). Significance of differences was determined by using 2-tailed Student t test, 1-way analysis of variance (ANOVA), 2-way ANOVA, or 1 sample Student t test. P values <.05 were considered significant (*P < .05, **P < .01, ***P < .001).
Results
Conditional genetic invalidation of Vps34 in the MK/platelet lineage
To generate a mouse model with conditional invalidation of Vps34 activity, exon 21 of the kinase domain of the Pik3c3 gene was flanked with LoxP sites (Pik3c3lox, B.B., and B.V., manuscript in preparation). Excision of exon 21 encoding the kinase domain of Vps34 specifically in the MK/platelet lineage was obtained by breeding Pik3c3lox/lox mice with Pf4-Cre transgenic mice to generate Pf4-Cre-Pik3c3lox/lox mice (referred to as Vps34 in Figures 1-6). Littermate Pik3c3lox/lox (referred to as wild-type [WT]) mice were used as controls. Platelet and MK lysates were analyzed by western blotting to assess the stability of the Vps34 protein lacking the exon 21 coding sequence. A dramatic reduction in the Vps34 protein expression level was observed specifically in MKs (84.3% ± 2.6%) and platelets (87.8% ± 1.5%) from Pf4-Cre-Pik3c3lox/lox mice (supplemental Figure 1A-B). Similarly, the level of Vps15, a Vps34 regulatory and associated protein kinase, was also strongly decreased in MKs and platelets from Pf4-Cre-Pik3c3lox/lox mice (supplemental Figure 1A). This correlates with a previous study reporting the requirement of Vps34 to maintain the protein complex integrity.26 Vps34 deletion had no impact on class I and II PI3K isoform expression in MKs and platelets (supplemental Figure 1A). Some residual lipid kinase activity (17.1% ± 4.1%) was detected in Vps34 immunoprecipitates from Vps34-deficient platelets (supplemental Figure 1C), suggesting efficient but incomplete Cre recombinase activity. Pf4-Cre-Pik3c3lox/lox mice were viable and fertile, developed normally with no apparent morphological abnormalities, and showed no signs of spontaneous bleeding.
Defective platelet production and granule distribution in Vps34-deficient platelets. (A) Whole blood platelet count was measured by using a HORIBA ABX Micros 60 analyzer (mean ± SEM; n = 38 mice for WT and 49 for Pf4-Cre-Pik3c3lox/lox mice [Vps34]); ***P < .001 vs WT according to 2-tailed Student t test) (left). Quantification of the percentage of mice with a mean platelet volume ranging from 4 to 7 µm3 and from 7 to 10 µm3 (mean ± SEM; n = 38 mice for WT and 49 for Pf4-Cre-Pik3c3lox/lox mice [Vps34]) (middle). TEM of resting platelets (right). Images are representative of 5 mice of each genotype. Scale bar represents 2 µm. (B) Mice were intravenously injected with a dylight488–anti-GPIbβ immunoglobulin derivative antibody. The percentage of labeled platelets in blood samples was measured at various time points after injection. (C) Thrombocytopenia in mice was induced by intraperitoneal injection of anti-GPIbα antibody (left). The platelet count was measured in blood samples collected 6 hours after injection (time = 0) and at various time points. The mouse serum TPO level was quantified by immunoassay (middle). Platelets were incubated with 50 ng/mL of TPO at the indicated times and after fixation with a rat antibody against the extracellular domain of Mpl and an anti-rat Alexa Fluor488 antibody (right). The graph is expressed as the percentage of the mean fluorescence intensity (MFI) resting (0) values after flow cytometry analysis (mean ± SEM; n = 4-6 mice of each genotype, *P < .05 vs WT according to 2-way ANOVA). (D) TEM of resting platelets. Images are representative of 5 mice of each genotype. Scale bar represents 1.5 µm. Arrows indicate α-granules. Platelet α- and dense (δ)-granule numbers and mean areas were measured on TEM images by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-tailed Student t test).
Defective platelet production and granule distribution in Vps34-deficient platelets. (A) Whole blood platelet count was measured by using a HORIBA ABX Micros 60 analyzer (mean ± SEM; n = 38 mice for WT and 49 for Pf4-Cre-Pik3c3lox/lox mice [Vps34]); ***P < .001 vs WT according to 2-tailed Student t test) (left). Quantification of the percentage of mice with a mean platelet volume ranging from 4 to 7 µm3 and from 7 to 10 µm3 (mean ± SEM; n = 38 mice for WT and 49 for Pf4-Cre-Pik3c3lox/lox mice [Vps34]) (middle). TEM of resting platelets (right). Images are representative of 5 mice of each genotype. Scale bar represents 2 µm. (B) Mice were intravenously injected with a dylight488–anti-GPIbβ immunoglobulin derivative antibody. The percentage of labeled platelets in blood samples was measured at various time points after injection. (C) Thrombocytopenia in mice was induced by intraperitoneal injection of anti-GPIbα antibody (left). The platelet count was measured in blood samples collected 6 hours after injection (time = 0) and at various time points. The mouse serum TPO level was quantified by immunoassay (middle). Platelets were incubated with 50 ng/mL of TPO at the indicated times and after fixation with a rat antibody against the extracellular domain of Mpl and an anti-rat Alexa Fluor488 antibody (right). The graph is expressed as the percentage of the mean fluorescence intensity (MFI) resting (0) values after flow cytometry analysis (mean ± SEM; n = 4-6 mice of each genotype, *P < .05 vs WT according to 2-way ANOVA). (D) TEM of resting platelets. Images are representative of 5 mice of each genotype. Scale bar represents 1.5 µm. Arrows indicate α-granules. Platelet α- and dense (δ)-granule numbers and mean areas were measured on TEM images by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-tailed Student t test).
Vps34 is critical for MK migration and granule biogenesis. (A) TEM of MKs from native bone marrow sections. Images are representative of 5 mice of each genotype. Scale bar represents 5 µm (upper images) and 1 µm (lower images). The α-granule number and mean area were quantified on 70-µm2 field of TEM images by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; **P < .01; ***P < .001 vs WT according to 2-tailed Student t test). (B) WT or Vps34-deficient (Vps34) MKs were exposed to a SDF1α gradient within the Dunn chamber. Migration paths over 6 hours of 5 representative MKs from 8 independent experiments in each graph were traced. The intersection of the x-axis and y-axis was taken to be the starting point of each cell path, whereas the source of the SDF1α was at the top. The accumulated distance and directionality from WT MKs in the presence of dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 or from Vps34-deficient (Vps34) MKs were analyzed by using the ImageJ software manual tracking plug-in (mean ± SEM; n = 30-50 MKs from 8 independent experiments; **P < .01 vs WT according to 1-way ANOVA). (C) Representative confocal images of immunostained native bone marrow. MKs and platelets are labeled by vWF staining (green). FABP4/A-FABP staining (red) labels sinusoid vessels. Nucleus staining was done by 4′,6-diamidino-2-phenylindole (blue). Scale bars represent 50 μm. The graph represents the percentage of platelets inside and outside the sinusoids (mean ± SEM; n = 20 images from 4 mice of each genotype, ***P < .001 vs WT according to 2-tailed Student t test).
Vps34 is critical for MK migration and granule biogenesis. (A) TEM of MKs from native bone marrow sections. Images are representative of 5 mice of each genotype. Scale bar represents 5 µm (upper images) and 1 µm (lower images). The α-granule number and mean area were quantified on 70-µm2 field of TEM images by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; **P < .01; ***P < .001 vs WT according to 2-tailed Student t test). (B) WT or Vps34-deficient (Vps34) MKs were exposed to a SDF1α gradient within the Dunn chamber. Migration paths over 6 hours of 5 representative MKs from 8 independent experiments in each graph were traced. The intersection of the x-axis and y-axis was taken to be the starting point of each cell path, whereas the source of the SDF1α was at the top. The accumulated distance and directionality from WT MKs in the presence of dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 or from Vps34-deficient (Vps34) MKs were analyzed by using the ImageJ software manual tracking plug-in (mean ± SEM; n = 30-50 MKs from 8 independent experiments; **P < .01 vs WT according to 1-way ANOVA). (C) Representative confocal images of immunostained native bone marrow. MKs and platelets are labeled by vWF staining (green). FABP4/A-FABP staining (red) labels sinusoid vessels. Nucleus staining was done by 4′,6-diamidino-2-phenylindole (blue). Scale bars represent 50 μm. The graph represents the percentage of platelets inside and outside the sinusoids (mean ± SEM; n = 20 images from 4 mice of each genotype, ***P < .001 vs WT according to 2-tailed Student t test).
Defective intracellular trafficking and PI3P production in Vps34-deficient MKs. (A-C) MK uptake of transferrin-Alexa Fluor546 (A) or fibrinogen-Alexa Fluor488 (B-C) was observed by confocal (A) or superresolution structured illumination microscopy (B-C) at different incubation time points. (A-B) Graphs represent fluorescence intensity quantified on a MK z-stack by ImageJ or Imaris software. (C) Representative 3-dimensional surface rendering of z-stacks acquired after 960 minutes of fibrinogen uptake are shown. Scale bar represents 5 µm. Graphs represent fibrinogen-positive structure number and volume quantified over time on 3-dimensional images using Imaris software (mean ± SEM; n = 10-60 MKs from 3 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-way ANOVA). (D-E) Fixed MKs stained with anti-clathrin, anti-EEA1, anti-Rab11, or anti-LAMP1 antibodies followed by corresponding secondary Alexa Fluor488 antibodies were observed by confocal microscopy. Live MKs were stained with LysoTracker Deep Red, fixed, and observed by confocal microscopy. Representative images of a z-stack are shown. Scale bar represents 5 µm. Graphs represent the structure number and area analyzed on a z-stack with ImageJ software (mean ± SEM; n = 30-50 MKs from 3 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-tailed Student t test). (F) PI3P mass assay performed on MKs as described in “Methods” (mean ± SEM; n = 5; **P < .01 vs WT according to 1-sample Student t test). (G) MKs stained with anti-PI3P and secondary Alexa Fluor488 antibodies were observed by confocal microscopy, and fluorescence intensity was quantified by using ImageJ software (mean ± SEM; n = 40 MKs from 3 mice of each genotype; ***P < .001 vs WT according to 2-tailed Student t test).
Defective intracellular trafficking and PI3P production in Vps34-deficient MKs. (A-C) MK uptake of transferrin-Alexa Fluor546 (A) or fibrinogen-Alexa Fluor488 (B-C) was observed by confocal (A) or superresolution structured illumination microscopy (B-C) at different incubation time points. (A-B) Graphs represent fluorescence intensity quantified on a MK z-stack by ImageJ or Imaris software. (C) Representative 3-dimensional surface rendering of z-stacks acquired after 960 minutes of fibrinogen uptake are shown. Scale bar represents 5 µm. Graphs represent fibrinogen-positive structure number and volume quantified over time on 3-dimensional images using Imaris software (mean ± SEM; n = 10-60 MKs from 3 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-way ANOVA). (D-E) Fixed MKs stained with anti-clathrin, anti-EEA1, anti-Rab11, or anti-LAMP1 antibodies followed by corresponding secondary Alexa Fluor488 antibodies were observed by confocal microscopy. Live MKs were stained with LysoTracker Deep Red, fixed, and observed by confocal microscopy. Representative images of a z-stack are shown. Scale bar represents 5 µm. Graphs represent the structure number and area analyzed on a z-stack with ImageJ software (mean ± SEM; n = 30-50 MKs from 3 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-tailed Student t test). (F) PI3P mass assay performed on MKs as described in “Methods” (mean ± SEM; n = 5; **P < .01 vs WT according to 1-sample Student t test). (G) MKs stained with anti-PI3P and secondary Alexa Fluor488 antibodies were observed by confocal microscopy, and fluorescence intensity was quantified by using ImageJ software (mean ± SEM; n = 40 MKs from 3 mice of each genotype; ***P < .001 vs WT according to 2-tailed Student t test).
Vps34 regulates stimulation-dependent PI3P production in platelets. (A) PI3P content was analyzed by mass assay in washed resting WT platelets treated for 1 hour with dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 and Vps34-deficient platelets (Vps34) (mean ± SEM; n = 3-5; **P < .01 vs WT according to 1-sample Student t test). (B) PI3P content from WT platelets treated for 1 hour with dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 and from Vps34-deficient platelets (Vps34) in resting or stimulated (CRP, 10 µg/mL; thrombin, 0.5 UI/mL) conditions was assessed by mass assay (mean ± SEM; n = 5; *P < .05; **P < .01 vs WT according to 2-way ANOVA). (C) HPLC analysis of the PI3P level of resting and stimulated (CRP, 10 µg/mL; thrombin, 0.5 IU/mL) 32 Pi-labeled platelets (mean ± SD; n = 2). (D) Vps34 was immunoprecipitated from resting or activated (CRP, 10 µg/mL; thrombin, 0.5 IU/mL) washed platelets and assayed for lipid kinase activity in vitro. Graph represents Vps34 activity (fold increase) normalized to the levels of immunoprecipitated kinase in each condition as assessed by immunoblot and densitometry analysis (mean ± SEM; n = 5; ***P < .001 vs resting according to 1-sample Student t test).
Vps34 regulates stimulation-dependent PI3P production in platelets. (A) PI3P content was analyzed by mass assay in washed resting WT platelets treated for 1 hour with dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 and Vps34-deficient platelets (Vps34) (mean ± SEM; n = 3-5; **P < .01 vs WT according to 1-sample Student t test). (B) PI3P content from WT platelets treated for 1 hour with dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 and from Vps34-deficient platelets (Vps34) in resting or stimulated (CRP, 10 µg/mL; thrombin, 0.5 UI/mL) conditions was assessed by mass assay (mean ± SEM; n = 5; *P < .05; **P < .01 vs WT according to 2-way ANOVA). (C) HPLC analysis of the PI3P level of resting and stimulated (CRP, 10 µg/mL; thrombin, 0.5 IU/mL) 32 Pi-labeled platelets (mean ± SD; n = 2). (D) Vps34 was immunoprecipitated from resting or activated (CRP, 10 µg/mL; thrombin, 0.5 IU/mL) washed platelets and assayed for lipid kinase activity in vitro. Graph represents Vps34 activity (fold increase) normalized to the levels of immunoprecipitated kinase in each condition as assessed by immunoblot and densitometry analysis (mean ± SEM; n = 5; ***P < .001 vs resting according to 1-sample Student t test).
Vps34 plays an important role in thrombosis via its kinase activity. (A) Tail bleeding time (n = 30 mice of each genotype) was measured as described in “Methods.” (B) The thrombotic response of mice to carotid injury after exposure to 7.5% ferric chloride for 3 minutes was assessed by a flow probe. Graph represents the percentage of mice without occlusion 30 minutes after injury (n = 15 mice of each genotype; P = .0002 according to 1-sample Student t test). (C-D) DiOC6-labeled platelets in mouse whole blood were perfused through collagen-coated microcapillaries at a physiological arterial shear rate of 500 s−1 (C) or at an arteriolar shear rate of 1500 s−1 (D). Scale bar represents 20 µm. The surface covered by fluorescent platelets and thrombus volume were analyzed using ImageJ software (mean ± SEM; n = 8 mice of each genotype; *P < .05; **P < .01 vs WT according to 2-tailed Student t test and 1-sample Student t test). (E) Healthy human donor whole blood was treated with 1 µM VPS34-IN1, 1 µM SAR405, or vehicle (dimethyl sulfoxide) for 1 hour. Then, DiOC6-labeled whole blood was perfused through collagen-coated microcapillaries at a physiological shear rate of 1500 s−1. Scale bar represents 20 µm. The surface covered by fluorescent platelets and thrombus volume were analyzed using ImageJ software (mean ± SEM; n = 3-5 healthy donors depending on the inhibitor; *P < .05; **P < .01 vs vehicle according to 2-way ANOVA and 1-sample Student t test).
Vps34 plays an important role in thrombosis via its kinase activity. (A) Tail bleeding time (n = 30 mice of each genotype) was measured as described in “Methods.” (B) The thrombotic response of mice to carotid injury after exposure to 7.5% ferric chloride for 3 minutes was assessed by a flow probe. Graph represents the percentage of mice without occlusion 30 minutes after injury (n = 15 mice of each genotype; P = .0002 according to 1-sample Student t test). (C-D) DiOC6-labeled platelets in mouse whole blood were perfused through collagen-coated microcapillaries at a physiological arterial shear rate of 500 s−1 (C) or at an arteriolar shear rate of 1500 s−1 (D). Scale bar represents 20 µm. The surface covered by fluorescent platelets and thrombus volume were analyzed using ImageJ software (mean ± SEM; n = 8 mice of each genotype; *P < .05; **P < .01 vs WT according to 2-tailed Student t test and 1-sample Student t test). (E) Healthy human donor whole blood was treated with 1 µM VPS34-IN1, 1 µM SAR405, or vehicle (dimethyl sulfoxide) for 1 hour. Then, DiOC6-labeled whole blood was perfused through collagen-coated microcapillaries at a physiological shear rate of 1500 s−1. Scale bar represents 20 µm. The surface covered by fluorescent platelets and thrombus volume were analyzed using ImageJ software (mean ± SEM; n = 3-5 healthy donors depending on the inhibitor; *P < .05; **P < .01 vs vehicle according to 2-way ANOVA and 1-sample Student t test).
Vps34 kinase activity regulates platelet secretion. (A) Kinetics of ATP secretion of washed platelets under resting or stimulated conditions (CRP, 1 µg/mL; thrombin, 0.1 IU/mL) were recorded by measuring the luminescence from the firefly luciferin-luciferase reaction by lumi-aggregometry using the Chrono-log aggregometer. Graphs represent the percentage of WT maximal secretion at 80 seconds (mean ± SEM; n = 6-15 mice of each genotype depending on the agonist; *P < .05; **P < .01; ***P < .001 vs WT according to 2-way ANOVA). (B) Kinetics of vWF secretion of washed platelets under resting or CRP (1 µg/mL) or thrombin (0.1 IU/mL) stimulated conditions was analyzed by enzyme-linked immunosorbent assay . The results are expressed as the fold increase compared with resting WT platelets (mean ± SEM; n = 6 mice of each genotype; *P < .05 vs resting WT according to 2-way ANOVA). The kinetics of ATP secretion (C) and vWF secretion (D) of washed platelets treated 1 hour with vehicle (dimethyl sulfoxide) or 1 μM VPS34-IN1 and stimulated with CRP (1 µg/mL) or thrombin (0.1 IU/mL) were analyzed as described above (mean ± SEM; n = 6; *P < .05 vs resting WT according to 2-way ANOVA). (E) Washed platelets were spread on a fibrinogen-coated surface for 20 minutes in the presence or absence of apyrase (2 IU/mL), and the platelet surface was measured using ImageJ software (mean ± SEM; n = 3 mice per genotype; **P < .01 vs WT according to 2-way ANOVA). (F) Whole blood from WT mice treated for 1 hour with dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 or from Pf4-Cre-Pik3c3lox/lox mice was perfused through a collagen-coated microcapillary in the presence of fluoxetine (25 µM) at a physiological shear rate of 1500 s−1. The serotonin content was quantified in plasma by immunoassay (mean ± SEM normalized by thrombus volume; n = 3-5 per condition; ***P < .001 vs WT according to 1-way ANOVA). (G) Unlabeled whole blood from WT (WT > WT) or Pf4-Cre-Pik3c3lox/lox (WT > Vps34) mice were perfused through collagen-coated microcapillaries at 1500 s−1 for 1 minute, and were then replaced by DiOC6-labeled WT whole blood perfused at the same shear rate. The surface covered by fluorescent platelets was analyzed by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; ***P < .001 vs WT > WT according to 2-way ANOVA test).
Vps34 kinase activity regulates platelet secretion. (A) Kinetics of ATP secretion of washed platelets under resting or stimulated conditions (CRP, 1 µg/mL; thrombin, 0.1 IU/mL) were recorded by measuring the luminescence from the firefly luciferin-luciferase reaction by lumi-aggregometry using the Chrono-log aggregometer. Graphs represent the percentage of WT maximal secretion at 80 seconds (mean ± SEM; n = 6-15 mice of each genotype depending on the agonist; *P < .05; **P < .01; ***P < .001 vs WT according to 2-way ANOVA). (B) Kinetics of vWF secretion of washed platelets under resting or CRP (1 µg/mL) or thrombin (0.1 IU/mL) stimulated conditions was analyzed by enzyme-linked immunosorbent assay . The results are expressed as the fold increase compared with resting WT platelets (mean ± SEM; n = 6 mice of each genotype; *P < .05 vs resting WT according to 2-way ANOVA). The kinetics of ATP secretion (C) and vWF secretion (D) of washed platelets treated 1 hour with vehicle (dimethyl sulfoxide) or 1 μM VPS34-IN1 and stimulated with CRP (1 µg/mL) or thrombin (0.1 IU/mL) were analyzed as described above (mean ± SEM; n = 6; *P < .05 vs resting WT according to 2-way ANOVA). (E) Washed platelets were spread on a fibrinogen-coated surface for 20 minutes in the presence or absence of apyrase (2 IU/mL), and the platelet surface was measured using ImageJ software (mean ± SEM; n = 3 mice per genotype; **P < .01 vs WT according to 2-way ANOVA). (F) Whole blood from WT mice treated for 1 hour with dimethyl sulfoxide (WT) or 1 μM VPS34-IN1 or from Pf4-Cre-Pik3c3lox/lox mice was perfused through a collagen-coated microcapillary in the presence of fluoxetine (25 µM) at a physiological shear rate of 1500 s−1. The serotonin content was quantified in plasma by immunoassay (mean ± SEM normalized by thrombus volume; n = 3-5 per condition; ***P < .001 vs WT according to 1-way ANOVA). (G) Unlabeled whole blood from WT (WT > WT) or Pf4-Cre-Pik3c3lox/lox (WT > Vps34) mice were perfused through collagen-coated microcapillaries at 1500 s−1 for 1 minute, and were then replaced by DiOC6-labeled WT whole blood perfused at the same shear rate. The surface covered by fluorescent platelets was analyzed by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; ***P < .001 vs WT > WT according to 2-way ANOVA test).
Mild microthrombopenia and platelet granule abnormalities in the absence of Vps34
Deletion of Vps34 in the MK/platelet lineage resulted in microthrombocytopenia of moderate intensity. As shown in Figure 1A, there was a significant reduction in the platelet count (22.4% ± 1.9%; P < .001) and in the mean platelet volume (6.18 ± 0.24 μm3 for Pf4-Cre-Pik3c3lox/lox mice vs 7.39 ± 0.22 μm3 for WT mice; P < .01, with a significant increase in mice with a mean platelet volume between 4 and 7 µm3). The expression levels of major platelet surface receptors (α2β1, αIIbβ3, GPVI, and functional GPIb-IX-V) were comparable in Pf4-Cre-Pik3c3lox/lox and WT platelets (supplemental Figure 1D, supplemental Videos 1-2).
Circulating platelet count and size are the net result of a balance between platelet production and clearance that can be evaluated by in vivo measurements of platelet count recovery after immune-induced depletion and platelet life span. As shown in Figure 1B-C, platelet life span was normal in Pf4-Cre-Pik3c3lox/lox mice, whereas platelet production was altered. Despite a normal platelet recovery kinetic after anti-GPIbα antibody–induced thrombocytopenia, a reduced number of platelets was produced in Pf4-Cre-Pik3c3lox/lox mice (Figure 1C). Accordingly, serum thrombopoietin (TPO) levels, which are classically inversely proportional to platelet count, were significantly elevated in Pf4-Cre-Pik3c3lox/lox mice (Figure 1C). However, increased TPO levels can also reflect abnormalities in clathrin-dependent endocytosis of its receptor, Mpl.19,27 Although total and surface expression of Mpl in resting WT and Vps34-deficient platelets were comparable (supplemental Figure 2A), we observed a significant decrease in Mpl endocytosis after 20 minutes of TPO stimulation in Vps34-deficient platelets (Figure 1C). This indicates that elevated serum TPO levels result from a decreased platelet count and defective Mpl endocytosis in platelets from Pf4-Cre-Pik3c3lox/lox mice. Overall, these findings show that Pf4-Cre-Pik3c3lox/lox mouse microthrombocytopenia is due to defective platelet production by MKs.
Morphological analysis by transmission electron microscopy (TEM) revealed an abnormal granule distribution in Vps34-deficient platelets (Figure 1D). These platelets displayed a 33.2% ± 3.1% reduction in the number and a 44.5% ± 3.4% increase in the size of α-granules compared with WT platelets (Figure 1D). Abnormalities of dense granules were also observed with a 44.8% ± 3.1% reduction in number compared with WT platelets (Figure 1D). We also found that fibrinogen internalization was significantly delayed in Vps34-deficient platelets (supplemental Figure 2B). Thus, the decreased efficiency of αIIbβ3/fibrinogen and Mpl/TPO endocytosis demonstrates an important contribution of Vps34 in clathrin-mediated endocytosis in platelets. Despite delayed endocytosis in Vps34-deficient platelets, α- or dense granule contents (vWF, fibrinogen, or serotonin) in Vps34-deficient platelets were similar to that of WT platelets (supplemental Figure 2C). Of note, platelet mitochondrial content and activity appeared normal in Vps34-deficient platelets (supplemental Figure 2D).
Overall, these data indicate that Vps34 specifically regulates platelet production, endocytosis, and granule homeostasis.
Vps34 is critical for MK migration and granule biogenesis
To gain insight into the mechanisms leading to the observed platelet phenotype, we then analyzed MK morphology and functions. As shown in Figure 2A and supplemental Figure 3A-C, resident bone marrow MKs and bone marrow–derived MKs from Pf4-Cre-Pik3c3lox/lox mice displayed similar numbers, sizes, ploidy levels, demarcation membrane systems, and proplatelet-producing capacities compared with WT MKs. MK ploidy levels and proplatelet extension were also not affected after pharmacological inhibition of Vps34 using the specific Vps34 inhibitor, VPS34-IN120 (supplemental Figure 3B-C). However, TEM of MKs in their native bone marrow showed that Vps34-deficient MKs display less (21.4% ± 3.7% reduction in number) but bigger (26.5% ± 2.4% increase in size) α-granules compared with WT MKs (Figure 2A), indicating that platelet granule defects originate from an abnormal biogenesis in MKs.
An important stage of megakaryopoiesis prior to platelet release into the circulation is MK migration from the proliferative osteoblastic niche to the capillary-rich vascular niche in response to an SDF1α gradient.28 We found that in vitro MK migration toward an SDF1α gradient on fibronectin, a major extracellular matrix component of the bone marrow,29 was significantly affected when Vps34 was absent or inhibited. Although the migration distance was comparable to that of WT MKs, deficiency or inhibition of Vps34 induced a significant reduction in megakaryocyte directionality toward SDF1α. The migration paths demonstrated that Vps34-deficient MKs moved over short distances before changing direction, indicating a lack of directional persistence toward the SDF1α gradient (Figure 2B). Immunostaining of bone marrow sections of intact femora showed that Vps34-deficient MKs more frequently released platelets outside the sinusoids directly into the bone marrow compartment than WT MKs (Figure 2C). Altogether, these findings are consistent with a critical role for Vps34 in regulating directional MK migration and the subsequent platelet release into sinusoid vessels. This strongly suggests that thrombocytopenia in Pf4-Cre-Pik3c3lox/lox mice is caused by the inability of mature MKs to reach bone marrow sinusoids, resulting in the observed ectopic platelet release.
Vps34 deletion affects intracellular trafficking and PI3P production in MKs
Both cell migration30 and granule biogenesis17,18 are regulated by intracellular trafficking mechanisms, such as endocytosis, recycling, and degradation. Besides normal transferrin receptor and αIIbβ3 integrin surface expression levels (supplemental Figure 3D), Vps34-deficient MKs displayed a significant reduction in transferrin and fibrinogen endocytosis after 20 minutes (Figure 3A-B). Moreover, large and less abundant fibrinogen-containing vesicles were observed over time by superresolution structured illumination microscopy in Vps34-deficient MKs compared with WT MKs (Figure 3C). These data show the important role of Vps34 in clathrin-mediated endocytosis in MKs as observed for platelets (Figure 1C; supplemental Figure 2B) and highlight its role in trafficking from the plasma membrane to granules in MKs. Consistent with these trafficking defects, we found, by confocal microscopy, a significant decreased number and increased size of clathrin-positive vesicles as well as an accumulation of large, early endosomes, as shown by the increased number (28.2% ± 2.7%) and size (16.7% ± 2.5%) of EEA1-positive vesicles in Vps34-deficient MKs (Figure 3D). Confocal analysis of LAMP1 and lysotracker labeling demonstrated a defect in early endosome-to-lysosome transitions in Vps34-deficient MKs compared with WT MKs. In addition, Rab11 labeling demonstrated a decrease in the number (43.9% ± 4.4%) and size (22.5% ± 1.3%) of recycling endosomes in Vps34-deficient MKs (Figure 3E). This is consistent with the elevated level of internalized transferrin at late time points (60 and 120 minutes) (Figure 3A) and point to impaired endosomal recycling. Altogether, these data demonstrate the existence of a general trafficking defect in Vps34-deficient MKs. This defect was not due to a loss of clathrin, early endosomal Rab5, late endosomal Rab7, or recycling endosomal Rab 11 GTPase expression (supplemental Figure 3E). Interestingly, the level of the Vps34 lipid product, PI3P, quantified by a specific mass assay23 and confocal microscopy using a specific anti-PI3P antibody, showed a significant reduction in Vps34-deficient MKs (Figure 3F-G). This reduction (from 30% to 40% compared with WT MKs) demonstrates the significant contribution of Vps34 to the level of PI3P but also suggests the existence of other enzymatic sources of PI3P in MKs. Overall, these data indicate a role for Vps34 in PI3P production and in the regulation of the endocytic/endosomal pathway in MKs with consequences for fibrinogen trafficking and, in turn, granule biogenesis.
In platelets, Vps34 is critical for the stimulation-dependent PI3P pool, but not for the basal PI3P pool
We next analyzed the impact of Vps34 deletion or inhibition in platelets using our mouse model and the Vps34-specific inhibitors (VPS34-IN1 and SAR405).20,21 We first focused on the production of PI3P by using both a specific mass assay23 quantifying the total PI3P amount and high-performance liquid chromatography (HPLC) analysis24 following short-term 32Pi-labeling to determine the acute changes in phosphoinositides after platelet stimulation. In resting platelets, the level of PI3P was modestly decreased (10.1% ± 1.3%) when Vps34 was deficient or pharmacologically inhibited with VPS34-IN1 (Figure 4A). Surprisingly, after CRP or thrombin stimulation, the inducible pool of PI3P was clearly affected (Figure 4B). HPLC analysis confirmed these defective responses (Figure 4C). No significant difference was observed in the amount of other lipid messengers, including phosphatidylinositol 3,4-bisphosphate, phosphatidylinositol 3,4,5-trisphosphate, and phosphatidic acid in resting or agonist-stimulated platelets (supplemental Figure 4). These data indicate that Vps34 is responsible for a small pool of basal PI3P in platelets and strongly involved in the agonist-dependent-pool of PI3P. To check whether Vps34 could be activated during platelet stimulation, we assayed its lipid kinase activity after immunoprecipitation. Acute WT platelet stimulations with thrombin or CRP resulted in a significant increase in Vps34 lipid kinase specific activity (Figure 4D). These data implicate a role for Vps34 activity in acute platelet stimulation.
Vps34 kinase activity regulates thrombus growth
We first tested the implication of Vps34 in platelet responses in vivo and found a normal tail bleeding time in Pf4-Cre-Pik3c3lox/lox mice (Figure 5A). The prothrombotic function of platelets tested after ferric chloride–induced mouse carotid injury was significantly decreased, with nearly 50% of Pf4-Cre-Pik3c3lox/lox mice protected against occlusive arterial thrombus formation (Figure 5B).
Ex vivo thrombus formation assay performed under physiological arterial or arteriolar wall shear rates of 500 and 1500 s−1, respectively, using Pf4-Cre-Pik3c3lox/lox mice blood perfused over a collagen surface showed that Vps34-deficient platelets, despite their ability to normally attach to collagen fibers (supplemental Figure 5A), formed significantly smaller thrombi compared with WT platelets (Figure 5C-D). When WT mouse blood was treated ex vivo with Vps34 inhibitors and then perfused on a collagen matrix, thrombus formation was affected to a similar extent as for Pf4-Cre-Pik3c3lox/lox mouse blood (supplemental Figure 5B). Importantly, when human blood was treated with Vps34 inhibitors, again a similar defect in thrombus growth was observed (Figure 5E). These data demonstrate that inhibition of Vps34 kinase activity in platelets, independently from its implication in MKs and platelet production, decreases platelet thrombus growth at arterial and arteriolar flows.
Vps34 kinase activity is required for controlled platelet secretion
To further analyze the thrombus formation defect, we performed aggregometry assays on platelets from Pf4-Cre-Pik3c3lox/lox mice and on human platelets treated with the Vps34 inhibitor. The mouse platelet aggregation responses to CRP, collagen, thrombin, thromboxane A2 analog, or adenosine 5′-diphosphate were not affected by Vps34 deficiency (supplemental Figure 6A). Platelet shape change and filopodia formation were also normal (supplemental Figure 6B) as well as thrombin or CRP-induced JON/A binding, reflecting normal αIIbβ3 integrin activation (supplemental Figure 6C). PI3P is known to contribute to the regulation of reduced NAD phosphate oxidase complex subunits,31 therefore, we measured the production of reactive oxygen species in response to CRP or thrombin. This production was not affected in Vps34-deficient platelets nor was thromboxane A2 production (supplemental Figure 6D-E), consistent with the fact that indomethacin treatment did not modify the thrombus growth difference between WT and Pf4-Cre-Pik3c3lox/lox mouse blood (supplemental Figure 6F). Aggregation responses of human platelets were also not affected following pharmacological inhibition of Vps34 (supplemental Figure 6G).
Interestingly, in the absence of Vps34 and despite a normal amount of granule content (supplemental Figure 2C), dense granule release (as assessed by adenosine triphosphate [ATP] secretion; Figure 6A) and α-granule release (as assessed by vWF secretion; Figure 6B) were significantly faster and exacerbated in response to acute platelet stimulation. This increased secretion response was also observed after treatment of WT platelets with the Vps34 inhibitor (Figure 6C-D). Consistent with this, Vps34-deficient platelets displayed an enhanced spreading on fibrinogen that was fully counteracted by hydrolysis of secreted ATP/adenosine 5′-diphosphate by the addition of apyrase (Figure 6E). Because low solute transport is important to maintain elevated concentrations of molecules secreted by platelets within the thrombus and to allow efficient thrombus growth,32,33 we checked whether the exacerbated secretion response could influence thrombus formation under arterial flow conditions. Measurement of the serotonin level in Pf4-Cre-Pik3c3lox/lox mouse whole blood or WT mouse whole blood treated with Vps34 inhibitor, recovered after perfusion on a collagen matrix, showed an increase compared to nontreated WT mice whole blood. This indicates that Vps34-deficient platelets or Vps34-inhibited WT platelets forming thrombi on collagen surfaces had exacerbated secretion, resulting in a higher level of secreted molecules in the circulation (Figure 6F). Accordingly, when DiOC6-labeled whole blood from WT mice was perfused under a physiological shear rate over preformed WT or Pf4-Cre-Pik3c3lox/lox thrombi, we observed that Vps34-deficient platelet thrombi were significantly less efficient in recruiting WT circulating platelets to allow thrombus growth (Figure 6G).
Taken together, these data show that Vps34 kinase activity controls the intensity and timing of platelet secretion, which likely affects thrombus growth under the arterial wall shear rate.
Discussion
By creating a mouse model of MK/platelet lineage-specific Vps34 deletion and using 2 unrelated Vps34 selective inhibitors, we revealed a dual role for Vps34 in MKs and in platelets. Pf4-Cre-Pik3c3lox/lox mice displayed a moderate microthrombocytopenia due to a platelet production defect rather than an abnormal platelet life span. Although nuclear and membrane maturation as well as proplatelet extension were normal in MKs when Vps34 was absent or inhibited, directional MK migration toward an SDF1α gradient was strongly affected. In the bone marrow, MKs migrate from the osteoblastic niche to the vascular niche to deliver platelets to the blood. Most likely as a result of this directional migration defect, Pf4-Cre-Pik3c3lox/lox mice showed an increased presence of platelets in the bone marrow outside the sinusoidal vessels, which is consistent with the mild microthrombocytopenia observed in these mice. Of note, this is also a case of patients with Wiskott-Aldrich syndrome and profilin 1–deficient mice where microthrombocytopenia has been reported to result from accelerated platelet turnover and premature platelet release into the bone marrow associated with defective MK migration toward the SDF1α gradient.34,35 Directed cell migration is a spatially organized process, governed by the cell’s ability to sense chemokine gradient and then transduce adequate signaling to polarize and move by chemotaxis, that depends on intracellular trafficking of cell surface receptors.30 SDF1α receptor–driven CXCR4 polarization has been shown to be important for MK-directed migration toward an SDF1α gradient.36 Thus, a defect that might explain the lack of directional migration of Vps34-deficient MKs is the herein documented defective trafficking (clathrin-mediated endocytosis and recycling) and the subsequent inadequate positioning of the SDF1α receptor, CXCR4, at the migration front. Unfortunately, the lack of efficient molecular tools to study CXCR4 in primary MKs did not allow us to evaluate this hypothesis. Because migration was assayed on a fibronectin matrix, impaired trafficking of other receptors, such as the fibronectin receptor α5β1 integrin, could contribute to the lack of MK directionality and in turn cause ectopic platelet release within the bone marrow.37 This study implements the short list of publications that highlight the important role of endocytosis in MKs and platelets.16,19 For instance, deletion of dynamin 2, a major actor of clathrin-mediated endocytosis, in MKs and platelets has been shown to strongly affect Mpl endocytosis associated with MK hyperplasia and myelofibrosis.19 Deletion of Vps34 in platelets also impacts Mpl and fibrinogen endocytosis, but with a somehow different associated phenotype (ie, moderated microthrombocytopenia without MK hyperplasia), probably due to a different degree of implication of the 2 proteins in endocytosis and their impact in other cellular processes.
The α- and dense- granules originate from multivesicular bodies/late endosome compartment where cargos derive from both a secretory pathway from the Golgi network and an endocytic pathway from the plasma membrane through highly regulated and still poorly known sorting processes.17,18 Thus, the aberrant granule biogenesis, highlighted by the disturbed fibrinogen uptake, is consistent with a general trafficking defect of Vps34-deficient MKs and platelets.
PI3P is an important lipid in the regulation of the endocytic pathway, and Vps34 has been proposed as the major PI3P-producing kinase in mammalian cells.4,5 Our results show that in MKs, Vps34 controls ∼40% of the PI3P level, which points to the contribution of other PI3K isoforms in the production of this lipid. Overall, our results show that Vps34 controls a PI3P pool that is important for the regulation of the endocytic and trafficking pathway in MKs. Although we observed a role for Vps34 and PI3P in autophagy in MKs (S.S. and C.V. unpublished observations), the trafficking defect shown in this study appears to be crucial in MK migration and granule biogenesis. This is consistent with the fact that Pf4-Cre-Atg7lox/lox mice, which exhibit impaired autophagy, have a normal platelet count, size, and ultrastructure.38 Interestingly, in platelets, the contribution of Vps34 to the production of the basal pool of PI3P is very limited (∼10%) when compared with the significant contribution of PI3KC2α that we recently described.39 Several routes of PI3P production probably account for maintaining basal levels of this important lipid, including Vps34 and mainly PI3KC2α, but also possibly 4- and/or 5-phosphatases acting on phosphatidylinositol 3,4-bisphosphate and/or phosphatidylinositol 3,4,5-trisphosphate.40 However, a significant fraction of stimulation-dependent PI3P production requires Vps34 activity as shown in platelets from Pf4-Cre-Pik3c3lox/lox mice and in WT platelets treated in vitro with Vps34 inhibitors. Accordingly, an increase in Vps34 kinase activity was observed following stimulation of WT platelets with G-protein coupled receptors or immunoreceptor tyrosine-based activation motif–linked receptors. Stimulation-dependent activation of Vps34 has also been suggested in insulin-stimulated hepatocytes,41 pointing toward a role for Vps34 during acute cellular responses.
Acute platelet activation can be triggered in vivo in a thrombosis model of carotid ferric chloride injury. Interestingly, the loss of Vps34 in platelets had a significant protective role against occlusive arterial thrombus formation. The decrease in thrombus growth at the arterial shear rate was confirmed in ex vivo thrombosis assays by using Pf4-Cre-Pik3c3lox/lox mouse blood perfused over a collagen surface and was reproduced by treating blood from WT mice or human donors with Vps34 inhibitors, thus highlighting the important role of Vps34 kinase activity in this process and showing that this effect is not a consequence of Vps34 functions in MKs.
Integrin αIIbβ3 activation and granule secretion are essential for the regulation of thrombus growth.42,43 The activation of αIIbβ3 integrin did not appear to be significantly altered in Vps34-deficient platelets, and Vps34-deficient washed platelet aggregation in suspension was normal. However, Vps34-deficient platelets showed an increased rate of secretion in response to several agonists in vitro and, importantly, ex vivo under arterial flow. Platelet secretion plays an important physiological role by discharging a variety of molecules that contribute to platelet recruitment for adequate thrombus growth and organization. Mathematical models and in vivo experiments have shown that the architecture of the thrombus (ie, a stable core of fully activated, densely packed platelets with an outer shell of less-activated, loosely packed platelets) is the result of overlapping agonist gradients within the platelet mass.33,44,45 Moreover, stratified granule secretion has recently been observed in the thrombus with fully degranulated platelets at the base of the thrombus and granule-containing platelets at the vessel lumen,46 which demonstrates the importance of secretion events in thrombus formation and organization. These data indicate that a fine-tuned spatial and temporal regulation of granule release, which is lost in the absence of platelet Vps34 activity, is crucial for normal platelet thrombus formation. Consistent with this, under arterial flow, we showed that Vps34-deficient platelets displayed an exacerbated secretion and were less efficient at recruiting circulating WT platelets to the growing thrombus. It is not clear at present how Vps34 and its product, PI3P, contribute to spatiotemporal platelet secretion, but it is most likely due to the recruitment of intracellular proteins that regulate granule fusion and/or secretion.
In conclusion, our study points to a dual function for Vps34 in MKs and platelets. In MKs, Vps34 controls the production of an important pool of PI3P and regulates clathrin-dependent endocytosis and trafficking, which in turn affects MK migration and subsequent platelet release in the blood as well as granule biogenesis. In platelets, Vps34 mainly controls the production of a stimulus-dependent pool of PI3P and platelet secretion, thereby impacting thrombus growth under arterial flow. This study provides new insights into the effects of Vps34 inhibitors on hemostasis and thrombosis that will have to be taken into account if they are used in therapy in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the personnel of the Anexplo animal facilities (US006/Centre Régional d'Exploration Fonctionnelle et de Ressources Expérimentales, Inserm/Université Paul Sabatier) for animal handling, Genotoul Imaging facilities (E. Vega and M. Zanoun [Inserm U1048] and B. Payre and I. Fourqaux [Centre de Microscopie Électronique Appliquée à la Biologie]), the cytometry facility of Inserm U1048 (A. Zakaroff-Girard and C. Pecher), and the lipidomic facility of Inserm U1048 (J. Bertrand-Michel and P. Lefaouder). The authors also thank all past and present members of the B.P. laboratory.
C.V. was supported by fellowships from Université Toulouse III and Société Francaise d’Hématologie. Work in the laboratory of B.P. was supported by Inserm and Fondation pour la Recherche Médicale grants (DPC20111122988 to M-P.G. and DEQ20170336737 to B.P.). B.P. is a scholar of the Institut Universitaire de France. Work in the laboratory of B.V. was supported by the Medical Research Council (G0700755), the UK Biotechnology and Biological Sciences Research Council (BB/M013278/1, BB/I007806), Cancer Research UK (C23338/A15965), the Ludwig Institute for Cancer Research, and the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Authorship
Contribution: C.V., M.L., and S.S. designed the research, performed experiments, and analyzed data; B.V. and B.B. generated Pik3c3lox mice; G.C. and B.P. designed and performed the phosphoinositide analysis; C.C. performed arterial thrombosis in vivo; J.V. performed the experiments; F.G.-I. and M.-P.G analyzed the data; and B.P. and S.S. wrote the article.
Conflict-of-interest disclosure: B.V. is consultant to Karus Therapeutics (Oxford, United Kingdom). The remaining authors declare no competing financial interests.
Correspondence: Sonia Severin, INSERM U1048 and Université Toulouse 3, I2MC, 31432 Toulouse Cedex 4, France; e-mail: sonia.severin@inserm.fr.
References
Author notes
C.V. and M.L. contributed equally to this work.
B.P. and S.S. are joint senior authors.

![Figure 1. Defective platelet production and granule distribution in Vps34-deficient platelets. (A) Whole blood platelet count was measured by using a HORIBA ABX Micros 60 analyzer (mean ± SEM; n = 38 mice for WT and 49 for Pf4-Cre-Pik3c3lox/lox mice [Vps34]); ***P < .001 vs WT according to 2-tailed Student t test) (left). Quantification of the percentage of mice with a mean platelet volume ranging from 4 to 7 µm3 and from 7 to 10 µm3 (mean ± SEM; n = 38 mice for WT and 49 for Pf4-Cre-Pik3c3lox/lox mice [Vps34]) (middle). TEM of resting platelets (right). Images are representative of 5 mice of each genotype. Scale bar represents 2 µm. (B) Mice were intravenously injected with a dylight488–anti-GPIbβ immunoglobulin derivative antibody. The percentage of labeled platelets in blood samples was measured at various time points after injection. (C) Thrombocytopenia in mice was induced by intraperitoneal injection of anti-GPIbα antibody (left). The platelet count was measured in blood samples collected 6 hours after injection (time = 0) and at various time points. The mouse serum TPO level was quantified by immunoassay (middle). Platelets were incubated with 50 ng/mL of TPO at the indicated times and after fixation with a rat antibody against the extracellular domain of Mpl and an anti-rat Alexa Fluor488 antibody (right). The graph is expressed as the percentage of the mean fluorescence intensity (MFI) resting (0) values after flow cytometry analysis (mean ± SEM; n = 4-6 mice of each genotype, *P < .05 vs WT according to 2-way ANOVA). (D) TEM of resting platelets. Images are representative of 5 mice of each genotype. Scale bar represents 1.5 µm. Arrows indicate α-granules. Platelet α- and dense (δ)-granule numbers and mean areas were measured on TEM images by using ImageJ software (mean ± SEM; n = 5 mice of each genotype; *P < .05; ***P < .001 vs WT according to 2-tailed Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/18/10.1182_blood-2017-04-781641/4/m_blood781641f1.jpeg?Expires=1763472281&Signature=lOTRFwjgPzIuC4KUQVlBa0-6CO1i-2T64bNcLQjVO9IqfjZVZdjHy7EHh-65e6S42uubd38YmcKuEtsxAhixjnlWnreUC7HiP9tMEXsVN9KxPi9gZ0ab8OFnHq5jB~BNp50OJigGQv43BT0fZrRCXSQtnmzRekoZe5I1d74xTmr1RNbdjA~eDgiQCYOwCnnXkzVxYW89-22bTpb2KzuIb4viamMNuE2i2fKV9O~4Z93eJVqLIphGMlQU2bUmi-W1JS8t4SegwDQXnjS8fNP984renel6JWJkh5jknYH09CtNAhMQhuKJXWz5ieIO-rC4Pi-ly~1FW3GtyS~~IGKXRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal