Key Points
SGN-CD19B is broadly active in vitro against malignant B-cell lines, including double-hit and triple-hit lymphoma cell lines.
SGN-CD19B shows significant antitumor activity in vivo in preclinical models of B-NHL and B-cell–derived acute lymphoblastic leukemia.
Abstract
Patients with relapsed/refractory B-cell malignancies such as non-Hodgkin lymphoma (B-NHL) or acute lymphoblastic leukemia have a poor prognosis. Despite measurable clinical activity with new targeted therapies, many patients do not achieve a complete or durable response suggesting an opportunity to improve upon existing therapies. Here we describe SGN-CD19B, a pyrrolobenzodiazepine (PBD)-based anti-CD19 antibody drug conjugate (ADC) being investigated for treatment of B-cell malignancies, which has improved potency compared with other ADCs. CD19-expressing tumor cells rapidly internalize SGN-CD19B, and the released PBD drug induces DNA damage, resulting in G2/M cell cycle arrest and cell death. SGN-CD19B demonstrated activity against a broad panel of malignant B-cell lines and induced durable regressions in mice bearing xenografts derived from these B-cell malignancies. A single dose of SGN-CD19B induced durable regressions at 300 μg/kg (3 μg/kg drug equivalents); combination with rituximab decreased the curative dose to 100 μg/kg (1 μg/kg drug equivalents). These doses are significantly lower than the level of drug required with other ADC payloads. In cynomolgus monkeys, SGN-CD19B effectively depleted CD20+ B lymphocytes in peripheral blood and lymphoid tissues confirming that SGN-CD19B is pharmacodynamically active at well-tolerated doses. In summary, preclinical studies show SGN-CD19B is a highly active ADC, which releases a DNA cross-linking agent rather than a microtubule inhibitor. The distinct mechanism of action, broad potency, and potential to combine with rituximab suggest that SGN-CD19B may offer unique clinical opportunities in B-cell malignancies. A phase 1 clinical trial is in progress to investigate the therapeutic potential of SGN-CD19B in relapsed/refractory B-NHL. This trial was registered at www.clinicaltrials.gov as #NCT02702141.
Introduction
Non-Hodgkin lymphoma (NHL) is the most common hematologic malignancy with an estimated 72 580 new cases diagnosed and 20 150 deaths occurring in 2016.1 The most prevalent form of B-cell–derived NHL (B-NHL) is diffuse large B-cell lymphoma (DLBCL). DLBCL is a heterogeneous lymphoid malignancy composed of distinct subtypes based on molecular signature and clinical outcome.2 At least one-third of DLBCL patients will fail frontline treatment with anthracycline-based chemotherapy regimens such as R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).3 Approximately 40% of DLBCL patients that relapse after frontline treatment do not respond to salvage therapy.4 Of those who are able to subsequently undergo third-line treatment, only 20% of patients achieve a response.5 The median overall survival for the remaining nonresponders is a mere 4 months, highlighting the need for new treatment approaches.
In this article, we describe the development of SGN-CD19B, an antibody drug conjugate (ADC) with improved potency to address the unmet need in DLBCL and other B-cell malignancies. SGN-CD19B targets CD19, a prevalent marker expressed on the surface of malignant B cells.6-8 CD19 has been clinically validated by multiple clinical trials with a diverse number of therapeutic approaches including naked and bispecific antibodies, ADCs, and chimeric antigen receptor–modified T cells (CAR-T cells).9,10 SGN-CD19B was preceded in the clinic by SGN-CD19A, a clinically active auristatin ADC, which also targets CD19.11,12 In contrast to SGN-CD19A and other ADCs in development for NHL, SGN-CD19B uses a pyrrolobenzodiazepine (PBD) dimer payload attached to the antibody using engineered cysteines.13 PBD dimers exert antitumor activity by cross-linking DNA.14 This mechanism is distinct from the microtubule inhibition employed by auristatin ADCs and suggests that SGN-CD19B may offer different clinical opportunities. Our results shown here demonstrate that SGN-CD19B is widely active against CD19-positive malignant B-cell lines and has compelling antitumor activity in vivo in preclinical models of B-NHL and B-cell–derived acute lymphoblastic leukemia (B-ALL). Preclinical studies also revealed that the antitumor activity of SGN-CD19B is augmented by rituximab, suggesting that SGN-CD19B may be used at lower doses in the clinic when combined with rituximab. The convincing antitumor activity, potential to combine with rituximab, and evidence for pharmacodynamic activity at well-tolerated doses provide a strong rationale for the clinical testing of SGN-CD19B in relapsed/refractory B-NHL.
Materials and methods
Cell lines and reagents
Cell lines were obtained from American Type Culture Collection (Manassas, VA) or Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and cultured in a tissue culture incubator at 37°C according to provider recommendations.
Humanized BU12 monoclonal antibody and generation of SGN-CD19B
The human CD19-selective antibody BU12 was generated by immunization of mice with the Burkitt lymphoma cell line EB4.30.15,16 A humanized version of this antibody was developed and found to have comparable antibody binding11 and potent cytotoxicity using auristatin drug linkers.11,17 Humanized BU12 was further modified to contain a cysteine at position 239 on the heavy chain enabling site-specific drug attachment of the PBD dimer (SGD-1882) via a maleimidocaproyl-valine-alanine drug linker as described previously.13
Determination of in vitro drug-induced cytotoxicity
For in vitro cytotoxicity assays, B-NHL and B-ALL cell lines were incubated with ADC in RPMI 1640 media supplemented with 10% heat-inactivated bovine serum (Gemini Bio Products, West Sacramento, CA) for 96 hours. Cell viability was measured with CellTiter-Glo (Promega, Madison, WI), and luminescence was determined using an Envision Xcite multiplate reader (PerkinElmer, Waltham, MA). In some studies, cells were first treated with ADC or unconjugated SGD-1882 and then processed for caspase-3/7 activity (Promega). Data were analyzed using GraphPad, and 50% inhibitory concentration (IC50) values were reported as the concentration of compound needed to yield a 50% reduction in viability compared with vehicle-treated cells.
Immunofluorescence microscopy
Ramos cells were plated on d-lysine–coated slides and bound with 200 ng/mL SGN-CD19B or nonbinding control at 4°C. Cells were fixed immediately after binding antibody or incubated at 37°C for 4 hours and then fixed with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) for 20 minutes at 4°C. Cells were then stained for 1 hour with anti-CD107A-AlexaFLuor488, followed by anti-human IgG-AlexaFluor647 (ThermoFisher, Waltham, MA). Between each antibody staining, cells were washed with Cytofix/Cytoperm Wash. Cells were mounted using Prolong Gold Antifade with 4′,6-diamidino-2-phenylindole mounting medium (ThermoFisher) and imaged using an Olympus IX83 inverted fluorescence microscope through a ×60 oil objective NA 1.35. Images were acquired with an Orca-Flash CMOS camera through CellSens Olympus software.
Cell cycle analyses
For cell cycle analysis, B-NHL cell lines were labeled for 30 minutes with bromodeoxyuridine (BrdU; BD Biosciences). Nascent DNA synthesis was detected using an anti-BrdU antibody (BD Biosciences), whereas total DNA content was detected with propidium iodide by flow cytometry with a FACSCalibur instrument (BD Biosciences).
Western blotting
Following treatment with ADC or unconjugated drug, cell lysates were prepared in lysis buffer [50 mM tris(hydroxymethyl)aminomethane (Tris) at pH 7.5 containing 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, protease inhibitor cocktail (Roche), and phosphatase inhibitor sets 1 and 2 (EMD Millipore, Billerica, MA)] and run on 4% to 20% gradient Tris-Gly minigels (ThermoFisher). After transfer, polyvinylidene difluoride membranes were blotted with rabbit polyclonal antibodies to cleaved poly(ADP-ribose)polymerase (PARP) or β-actin (Cell Signaling Technology, Danvers, MA). Histone 2AX (H2AX) phosphorylation was evaluated by western blot according to protocols from AbCam (Cambridge, MA). After cell lysis, histones were extracted as described,14 run on 4% to 12% Bis-Tris NuPAGE gels (ThermoFisher), transferred onto nitrocellulose membranes, and blotted with anti-H2AX rabbit polyclonal (AbCam) and anti-phospho-H2AX mouse monoclonal (EMD Millipore) antibodies. After washing, blots were incubated with appropriate horseradish peroxidase–conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA), and signal was detected using chemiluminescence (Supersignal West Pico; ThermoFisher).
Assessment of SGN-CD19B in in vivo models of B-NHL and B-ALL
All animal experiments were conducted in a facility accredited by the Association for Assessment of Laboratory Animal Care under Institutional Animal Care and Use Committee guidelines and appropriate animal research approval. For B-NHL models, female C.B-17 SCID mice (Harlan, Indianapolis, IN) were implanted with 5 × 106 cells (WSU-DLCL2, Ramos, and DoHH2) subcutaneously in the flank. Tumor growth was monitored throughout the course of the study with bilateral vernier caliper measurements, and mean tumor volumes were calculated using the following equation: 0.5 × (length × width2). When tumors reached ∼100 mm3, mice were randomly assigned to receive SGN-CD19B or a nonbinding control ADC. For combination studies, mice were given a suboptimal dose of SGN-CD19B (100 μg/kg, single dose) alone or in combination with rituximab (10 mg/kg, every fourth day for 4 injections). For the disseminated model of disease, 1 × 106 NALM-6 cells were injected IV into the lateral tail vein. Animals were observed and euthanized upon evidence of significant progressive disease (eg, hind limb paralysis, weight loss of >15%). Treatment with test compounds occurred 7 days after injection. Data were plotted and analyzed using the log-rank (Mantel Cox) test (GraphPad).
Assessment of B-lymphocyte depletion in cynomolgus monkeys
All studies were conducted at a research facility accredited by the Association for Assessment of Laboratory Animal Care. Animals were administered either control article or SGN-CD19B once by IV bolus injection at dose levels of 0, 10, 30, 100, 300, or 600 μg/kg. Blood was collected from all available animals prior to the initiation of dosing and 4 days, 3 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks postdose. CD20+ B lymphocytes, CD3+ T lymphocytes, CD3+/CD4 T-helper lymphocytes, CD3+/CD8+ T-cytotoxic lymphocytes, CD3−/CD16+ natural killer cells, and CD3−/CD14+ monocytes were evaluated via flow cytometry.
Histological evaluation of B-lymphoid depletion in the spleen was conducted as part of a Good Laboratory Practice single dose study of SGN-CD19B in cynomolgus monkeys. Animals were administered either control article or SGN-CD19B once by IV bolus injection at dose levels of 0, 30, 100, or 250 μg/kg. Scheduled necropsies were conducted 10 days, 8 weeks, and 16 weeks postdose. From each animal, 2 sections of spleen were collected for immunohistochemistry. The samples were fixed in formalin for 48 to 72 hours, embedded in paraffin, and immunohistochemically stained with antibodies directed against CD19, CD20, Ki67A, and appropriate isotype control antibodies. A qualitative analysis of the frequency and intensity of this staining was performed by a veterinary pathologist certified by the American College of Veterinary Pathologists.
Results
In vitro cytotoxicity of SGN-CD19B against CD19+ malignant B cells
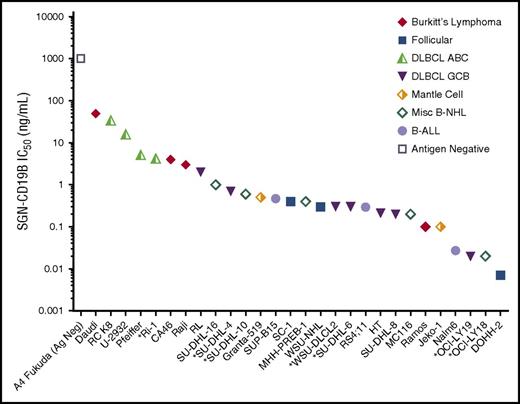
The in vitro cytotoxicity of SGN-CD19B was evaluated across a broad panel of B-NHL and B-ALL cell lines. A summary of the in vitro data is shown in Figure 1. Cell lines are ranked based on IC50 values. Tumor type and subtypes are indicated with distinct symbols. Results show SGN-CD19B is widely active against CD19+ tumor cell lines, with the majority of cell lines displaying robust sensitivity at or below 1 ng/mL. Lymphoma cell lines with MYC, BCL2, and/or BCL6 rearrangements,18 indicated by an asterisk in Figure 1, were among the cells that showed high sensitivity to SGN-CD19B. We did not see a strong correlation between CD19 expression levels and IC50 values for SGN-CD19B (supplemental Figure 1, available on the Blood Web site). However, when we examined the relative sensitivity of the cell line panel to SGD-1882, the active drug released by proteolytic cleavage of SGN-CD19B, we discovered Burkitt lymphoma cell lines and DLBCL cell lines of the activated B-cell (ABC) subtype19 are inherently less sensitive to SGD-1882 (K.A.G., unpublished data). Hence, the higher IC50 values seen with DLBCL-ABC and Burkitt lymphoma cell lines align with their relative sensitivity to SGD-1882. RNA sequencing identified several candidate genes that may regulate PBD activity, and work is in progress to understand the molecular pathways responsible for drug sensitivity. Despite varied sensitivity to SGD-1882, SGN-CD19B displayed broad activity across B-NHL cell lines. As expected, no detectable activity was seen using an antigen-negative cell line (Figure 1, open square) or nonbinding control ADCs (data not shown).
In vitro cytotoxicity of SGN-CD19B on malignant B-cell lines. SGN-CD19B activity was evaluated across a broad panel of B-NHL and B-ALL cell lines using a 96-hour cell viability assay. Malignant B-cell lines are plotted based on IC50 values (y-axis). The x-axis shows ranking of SGN-CD19B sensitivity from lowest to highest. Unique symbols denote tumor type or subtype. Double-hit or triple-hit lymphoma cell lines, as reported in the literature,18 are indicated on the x-axis using an asterisk.
In vitro cytotoxicity of SGN-CD19B on malignant B-cell lines. SGN-CD19B activity was evaluated across a broad panel of B-NHL and B-ALL cell lines using a 96-hour cell viability assay. Malignant B-cell lines are plotted based on IC50 values (y-axis). The x-axis shows ranking of SGN-CD19B sensitivity from lowest to highest. Unique symbols denote tumor type or subtype. Double-hit or triple-hit lymphoma cell lines, as reported in the literature,18 are indicated on the x-axis using an asterisk.
SGN-CD19B mechanism of action
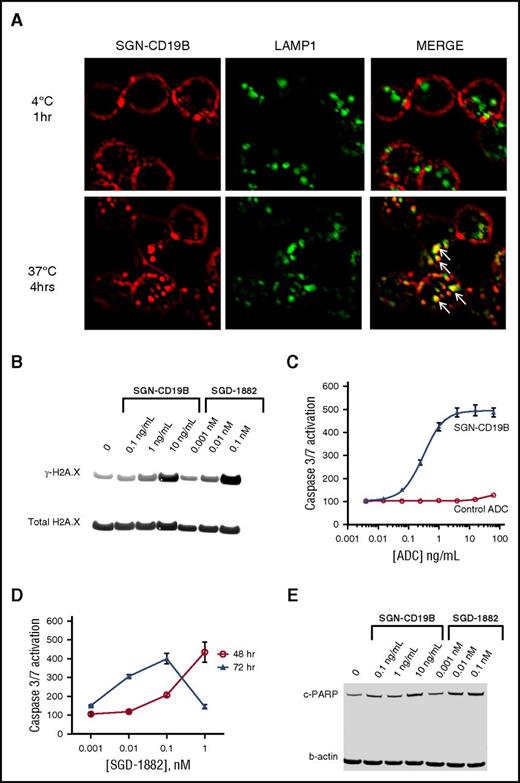
We used immunofluorescent microscopy to monitor the binding and internalization of SGN-CD19B in CD19-expressing tumor cells. As depicted in Figure 2A, SGN-CD19B binds to CD19 on the cell surface of lymphoma cells resulting in internalization of the ADC-CD19 complex and trafficking through the endocytic pathway. Localization at 4°C shows SGN-CD19B (red) is detectable on the cell surface and can be distinguished from LAMP-1 (green) staining in lysosomes (Figure 2A, upper right). In contrast, after 4 hours of incubation at 37°C, SGN-CD19B is detectable inside the cell where it colocalizes with LAMP-1 in lysosomes (Figure 2A, lower right), consistent with trafficking through the endocytic pathway.
Mechanism of action of SGN-CD19B. (A) Cell surface and intracellular localization of SGN-CD19B in WSU-DLCL2 lymphoma cells was detected by fluorescence microscopy. SGN-CD19B binds to CD19 on the cell surface following treatment of 1 hour at 4°C and is distinguishable from LAMP-1 (upper panels). Following a 4-hour incubation at 37°C (t = 4 hours), SGN-CD19B shows evidence of internalization (lower left) and colocalization with LAMP-1 (lower right). Arrows indicate areas of colocalized signal (yellow) observed in the merged image (lower right). Original magnification ×600. WSU-DLCL2 lymphoma cells were incubated with SGN-CD19B (ng/mL) or SGD-1882 (nM) for 48 or 72 hours prior to western blot analyses with antibodies specific for γ-H2AX (B) or cleaved PARP (E). Antibodies recognizing total H2AX (B) or β-actin (E) were used to confirm equivalent protein loading. (C) Caspase 3/7 activation was assessed by treating cells with SGN-CD19B (blue closed triangles) or a nonbinding control ADC (red open circles) for 72 hours prior to evaluation of caspase 3/7 activity. (D) Caspase 3/7 activation following treatment of cells with free drug (SGD-1882) at 48 hours (red open circles) and 72 hours (blue closed triangles).
Mechanism of action of SGN-CD19B. (A) Cell surface and intracellular localization of SGN-CD19B in WSU-DLCL2 lymphoma cells was detected by fluorescence microscopy. SGN-CD19B binds to CD19 on the cell surface following treatment of 1 hour at 4°C and is distinguishable from LAMP-1 (upper panels). Following a 4-hour incubation at 37°C (t = 4 hours), SGN-CD19B shows evidence of internalization (lower left) and colocalization with LAMP-1 (lower right). Arrows indicate areas of colocalized signal (yellow) observed in the merged image (lower right). Original magnification ×600. WSU-DLCL2 lymphoma cells were incubated with SGN-CD19B (ng/mL) or SGD-1882 (nM) for 48 or 72 hours prior to western blot analyses with antibodies specific for γ-H2AX (B) or cleaved PARP (E). Antibodies recognizing total H2AX (B) or β-actin (E) were used to confirm equivalent protein loading. (C) Caspase 3/7 activation was assessed by treating cells with SGN-CD19B (blue closed triangles) or a nonbinding control ADC (red open circles) for 72 hours prior to evaluation of caspase 3/7 activity. (D) Caspase 3/7 activation following treatment of cells with free drug (SGD-1882) at 48 hours (red open circles) and 72 hours (blue closed triangles).
To assess SGN-CD19B-induced cytotoxicity following internalization, we analyzed cells for evidence of DNA damage, induction of apoptosis, and changes in the cell cycle profile. PBDs act by cross-linking DNA and inducing sequence-specific DNA lesions,20,21 which can be readily monitored by phosphorylation of H2AX at serine 139 (γ-H2AX).14 As illustrated in Figure 2B, WSU-DLCL2 cells treated for 48 hours with SGN-CD19B or SGD-1882 show dose-dependent increases in γ-H2AX levels, indicative of DNA damage. When DNA repair mechanisms fail, the pathway to apoptosis and cell death is initiated. Figure 2C shows SGN-CD19B induces dose-dependent activation of caspase 3/7, whereas the nonbinding control ADC has no effect. Caspase 3/7 activation is also seen when cells are treated with SGD-1882 (Figure 2D). Likewise, cleaved PARP, a hallmark of caspase-mediated cellular apoptosis, was significantly increased 72 hours after treatment with SGN-CD19B or SGD-1882 (Figure 2E).
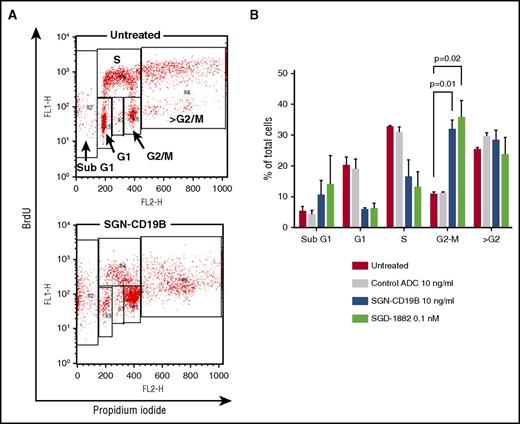
Next, we used BrdU and propidium iodide incorporation to analyze the cell cycle profile of WSU-DLCL2 cells following treatment with SGN-CD19B and SGD-1882. The results, illustrated in Figure 3, show SGN-CD19B- and SGD-1882-treated cells arrested in the G2/M phase of the cell cycle in a dose-dependent manner, whereas a nonbinding control ADC had no effect on cell cycle distribution. In addition, we saw an increased number of cells in SubG1, indicative of apoptosis. Taken together, these data suggest that the PBD drug released by SGN-CD19B induces DNA damage, resulting in G2/M cell cycle arrest and cell death.
SGN-CD19B induces cell cycle arrest. (A) Representative flow cytometry plots for untreated cells (top) and SGN-CD19B-treated cells (bottom). Cells were labeled with BrdU (y-axis) and propidium iodide (x-axis). Gates are indicated with boxes. (B) Cell cycle profile at 48-hour time point. Cells were untreated or incubated with control ADC (10 ng/mL), SGN-CD19B (10 ng/mL), or 0.1 nM PBD. Bar graphs represent results from 3 different experiments. Accumulation of cells in G2/M phase following treatment with SGN-CD19B or free PBD drug (SGD-1882) is statistically significant when compared with untreated and control ADC groups.
SGN-CD19B induces cell cycle arrest. (A) Representative flow cytometry plots for untreated cells (top) and SGN-CD19B-treated cells (bottom). Cells were labeled with BrdU (y-axis) and propidium iodide (x-axis). Gates are indicated with boxes. (B) Cell cycle profile at 48-hour time point. Cells were untreated or incubated with control ADC (10 ng/mL), SGN-CD19B (10 ng/mL), or 0.1 nM PBD. Bar graphs represent results from 3 different experiments. Accumulation of cells in G2/M phase following treatment with SGN-CD19B or free PBD drug (SGD-1882) is statistically significant when compared with untreated and control ADC groups.
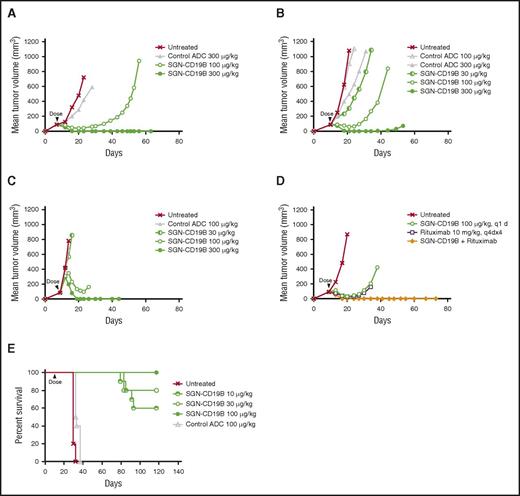
SGN-CD19B activity in vivo
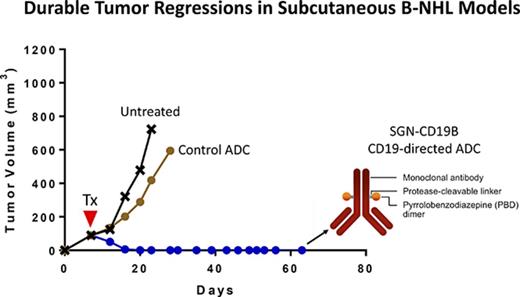
SGN-CD19B was evaluated in preclinical models of B-cell malignancies including 3 subcutaneous models of B-NHL and 1 disseminated model of B-ALL. Among the B-NHL models, we selected WSU-DLCL2, Ramos, and DoHH2 cell lines, which are derived from DLBCL, Burkitt, and follicular lymphoma, respectively. Figure 4A-C shows that treatment of mice bearing subcutaneous lymphoma xenografts with a single dose of SGN-CD19B at 100 μg/kg results in significant tumor growth delay when compared with the control ADC at the same dose. At a higher dose of 300 μg/kg, mice achieved durable complete regressions as are defined by a lack of palpable tumor through the end of the experiment. Remarkably, the antitumor activity of SGN-CD19B at 100 μg/kg could be further improved by combining it with rituximab. As illustrated in Figure 4D, SGN-CD19B induced durable tumor regressions in follicular lymphoma at the lower dose of 100 μg/kg when it was combined with rituximab. In contrast, monotherapy treatment of rituximab or SGN-CD19B at comparable doses resulted in transient regressions that were not durable (Figure 4D). The improved activity seen with SGN-CD19B plus rituximab was also observed in DLBCL (supplemental Figure 2) and has yet to be tested in Burkitt lymphoma models.
In vivo activity of SGN-CD19B. Xenograft models for B-NHL were established by subcutaneous injection of WSU-DLCL2 (A), Ramos (B), and DoHH2 (C) cell lines, which are derived from DLBCL, follicular lymphoma, and Burkitt lymphoma, respectively. SGN-CD19B or control ADC was administered at a single dose as indicated by the arrowhead when tumor volume reached 100 mm3. Tumor delays occurred at 100 μg/kg dose, and complete durable regressions were achieved at the higher dose of 300 μg/kg. (D) DoHH2 (follicular lymphoma) xenografts were treated with 100 μg/kg of SGN-CD19B (single dose) in the presence or absence of 10 mg/kg of rituximab (every fourth day for 4 injections). Durable cures were attained in 100% of mice treated with SGN-CD19B plus rituximab. (E) A disseminated disease model for ALL was established by injecting 5 × 106 NALM-6 cells per mouse IV into female SCID mice (10 mice per group). Treatment of NALM-6 tumor-bearing mice was started 7 days post tumor implant only once at the doses indicated. Mice treated with ≥10 μg/kg of SGN-CD19B showed significantly improved survival out to >115 days, whereas mice in the untreated or control groups survived <40 days.
In vivo activity of SGN-CD19B. Xenograft models for B-NHL were established by subcutaneous injection of WSU-DLCL2 (A), Ramos (B), and DoHH2 (C) cell lines, which are derived from DLBCL, follicular lymphoma, and Burkitt lymphoma, respectively. SGN-CD19B or control ADC was administered at a single dose as indicated by the arrowhead when tumor volume reached 100 mm3. Tumor delays occurred at 100 μg/kg dose, and complete durable regressions were achieved at the higher dose of 300 μg/kg. (D) DoHH2 (follicular lymphoma) xenografts were treated with 100 μg/kg of SGN-CD19B (single dose) in the presence or absence of 10 mg/kg of rituximab (every fourth day for 4 injections). Durable cures were attained in 100% of mice treated with SGN-CD19B plus rituximab. (E) A disseminated disease model for ALL was established by injecting 5 × 106 NALM-6 cells per mouse IV into female SCID mice (10 mice per group). Treatment of NALM-6 tumor-bearing mice was started 7 days post tumor implant only once at the doses indicated. Mice treated with ≥10 μg/kg of SGN-CD19B showed significantly improved survival out to >115 days, whereas mice in the untreated or control groups survived <40 days.
To assess the activity of SGN-CD19B in B-ALL, a disseminated model was developed using NALM-6 tumor cells where the median survival of untreated mice is usually <30 days post tumor cell implantation. Results shown in Figure 4E reveal treatment with the nonbinding control ADC minimally prolonged the median survival. In contrast, treatment with SGN-CD19B provided a significant survival benefit. One hundred percent of mice treated at 100 μg/kg survived to the end of study (day 117). Likewise, mice treated with 30 μg/kg of SGN-CD19B showed 80% survival, and mice treated with the lowest dose of 10 μg/kg showed 60% survival. These results collectively demonstrate SGN-CD19B has significant antitumor activity in B-NHL and B-ALL xenograft models that is dose dependent and immunologically specific. It is also notable that mice treated with SGN-CD19B did not show any signs of toxicity such as weight loss, which was evaluated concurrently with antitumor activity (supplemental Figure 3).
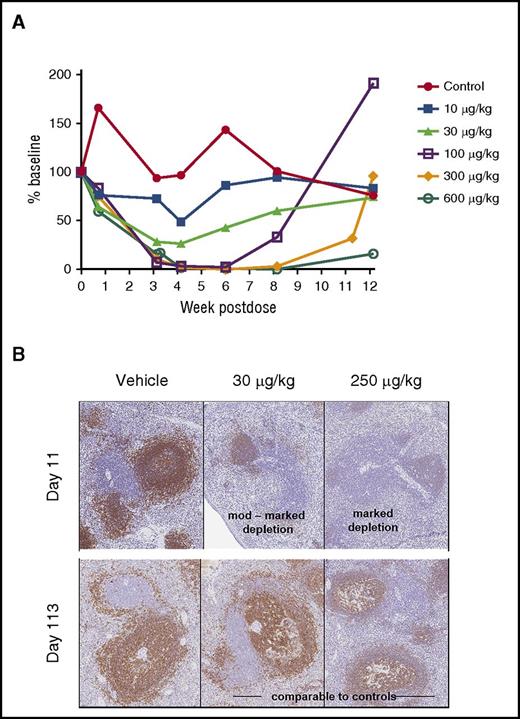
Next, we evaluated SGN-CD19B-mediated depletion of normal B cells in conjunction with nonhuman primate (NHP) toxicology studies. Results presented in Figure 5A show SGN-CD19B treatment resulted in dose-dependent decreases in circulating CD20+ B cells at dose levels ≥10 μg/kg. Complete B-lymphocyte depletion was attained at 100 μg/kg. Although response duration varied with dose level, all but the 600 μg/kg dose groups showed evidence of complete recovery by the end of study. Histological findings presented in Figure 5B show congruent B-lymphoid depletion in the spleen at doses ≥30 μg/kg. The moderate to marked germinal center depletion, which was evident by day 11, is followed by complete recovery (day 113) (Figure 5B). Depletion of normal B cells in circulation and in tissue confirms that SGN-CD19B is pharmacodynamically active at well-tolerated doses. The other major target organ identified was bone marrow, which showed decreased cellularity that correlated with a reduction in circulating red and white blood cells. This change showed evidence of recovery by the end of study.
CD20+B-cell depletion in NHPs. (A) CD20+ B cells were measured in serum over time (CD20+ B-lymphocytes mean). SGN-CD19B results in dose-dependent depletion of CD20+ B cells compared with control. Treatment groups are indicated by distinct symbols: vehicle control (red closed circles); 10 μg/kg (blue closed squares); 30 μg/kg (light green closed triangles); 100 μg/kg (purple open squares); 300 μg/kg (orange closed diamonds); and 600 μg/kg (green open circles). (B) Immunohistochemical staining of CD20+ B cells in spleen at day 11 and 113. Comparison is shown for untreated (left), 30 μg/kg SGN-CD19B (middle), or 250 μg/kg SGN-CD19B (right). At day 11, monkeys treated with 30 μg/kg or 250 μg/kg of SGN-CD19B showed moderate (upper middle) to marked (upper right) depletion. Recovery was evident by day 113 (lower middle and right panels). Original magnification ×10.
CD20+B-cell depletion in NHPs. (A) CD20+ B cells were measured in serum over time (CD20+ B-lymphocytes mean). SGN-CD19B results in dose-dependent depletion of CD20+ B cells compared with control. Treatment groups are indicated by distinct symbols: vehicle control (red closed circles); 10 μg/kg (blue closed squares); 30 μg/kg (light green closed triangles); 100 μg/kg (purple open squares); 300 μg/kg (orange closed diamonds); and 600 μg/kg (green open circles). (B) Immunohistochemical staining of CD20+ B cells in spleen at day 11 and 113. Comparison is shown for untreated (left), 30 μg/kg SGN-CD19B (middle), or 250 μg/kg SGN-CD19B (right). At day 11, monkeys treated with 30 μg/kg or 250 μg/kg of SGN-CD19B showed moderate (upper middle) to marked (upper right) depletion. Recovery was evident by day 113 (lower middle and right panels). Original magnification ×10.
Discussion
A number of clinically active ADCs are in development for B-cell NHL, including denintuzumab mafodotin (SGN-CD19A), polatuzumab vedotin (RG7596), inotuzumab ozogamicin (INO; CMC-544), and IMGN529.22,23 ADCETRIS (brentuximab vedotin), approved for relapsed classical Hodgkin lymphoma and systemic anaplastic large cell lymphoma,24 also shows significant activity in DLBCL.25 Many of the ADCs tested in relapsed/refractory DLBCL have shown single agent objective response rates of between 40% and 50%, but response durability is suboptimal, possibly owing to the fact that many patients achieved partial rather than complete responses. SGN-CD19A was an exception, demonstrating an objective response rate of 38%, complete response rate of 23% and a median duration of response of 10.9 months in relapsed and refractory DLBCL patients.12 Other CD19-directed therapies show promise in B-NHL such as blinatumomab, the bispecific CD19-directed CD3 T-cell engager,26 and KTE-19, a CD19-directed CAR-T cell.27 Blinatumomab shows reasonable activity in relapsed/refractory B-NHL,26 yet the requirement for continuous infusion and associated neurologic toxicities are notable drawbacks.28,29 CD19-directed CAR-T cell therapies have demonstrated promising responses in B-cell malignancies30 and will likely have a place in the future; however, technical challenges in manufacturing may limit their broad applicability to all relapsed/refractory patients.31-33
Here we describe the preclinical evaluation of SGN-CD19B and highlight notable features that distinguish it from other ADCs in development for B-NHL and B-ALL. These features include potency, mechanism of action, linker stability, and uniformity of drug load. Our in vivo studies show SGN-CD19B induces durable tumor regressions and improved survival at doses ≤300 μg/kg. This dose equates to an active dose of ≤3 μg/kg drug equivalents, which is 10- to 100-fold lower than the active dose required with other ADC payloads including auristatin,34 maytansine,35 and calicheamicin ADCs.36 Mechanistically, PBDs induce cell death by directly cross-linking DNA whereas auristatin37 and maytansine38 act by inhibiting microtubules. The site-specific cross-linking of PBD dimers in the minor groove of DNA produces minimal DNA distortion, potentially enabling evasion of DNA repair responses.21,39 This is distinct from calicheamicin, which induces DNA strand breaks, rather than cross-linking DNA.40 The distinct mechanism and properties of PBD dimers appear to confer activity in multidrug resistant (MDR+) tumor cells. For instance, preclinical studies on SGN-CD33A, a CD33-directed ADC studied in myeloid malignancies,41 revealed activity against MDR+ primary acute myeloid leukemia tumor samples.14 Recently, other PBD ADCs have entered the clinic, including ADCT-402, which also targets CD19. Although ADCT-402 and SGN-CD19B use structurally similar PBD dimers, preclinical data indicate ADCT-402 is 3- to 10-fold less potent than SGN-CD19B in comparable tumor models.42
We anticipate that an ADC with enhanced potency will be advantageous in lymphomas and solid tumors where tumor penetration is more of a barrier than it is with leukemia. Therefore, to assess the pharmacodynamic activity of SGN-CD19B in vivo, we evaluated the depletion of CD20+ B cells in circulating blood and lymphoid organs in conjunction with NHP safety studies. We detected dose-dependent depletion of circulating CD20-positive B cells at ≥10 μg/kg, and full depletion of CD20-positive B cells was evident by 100 μg/kg. In germinal centers of lymphoid follicles, we observed significant B-cell depletion at doses at low as 30 μg/kg. The appreciable B-cell depletion at relatively low drug doses suggests SGN-CD19B has the potential to deliver antitumor activity at doses that will be tolerated in humans. In the phase 1 dose escalation study of SGN-CD33A, the most common grade 3 adverse events were febrile neutropenia, thrombocytopenia, and anemia.41 These adverse events are consistent with on-target myelosuppression because of CD33 expression on myeloid progenitors. Although nontargeted myelosuppression is possible with ADCs, we anticipate a lesser degree of myelosuppression with SGN-CD19B relative to SGN-CD33A because of the fact that CD19 expression is restricted to the B-cell lineage.
Although SGN-CD19A and SGN-CD19B both target CD19, we expect these 2 ADCs may have different clinical opportunities in B-cell malignancies. SGN-CD19B demonstrates consistently potent preclinical activity across a wide variety of malignant B-cell lines (Figure 1), suggesting that it may perform well under conditions where tumor expression of CD19 is low or heterogeneous or when a patient’s tumor is characterized by poor prognostic features (ie, double-hit lymphoma). Aside from potency, the cytotoxic drugs released by SGN-CD19A and SGN-CD19B have different biophysical properties and induce cell death by distinct mechanisms of action, which will likely result in different toxicity and efficacy profiles. cys-mcMMAF, the drug released by SGN-CD19A,43 has a low octanol-water partition coefficient (Jocelyn Setter, unpublished observations), rendering it far less permeable to the plasma membrane and thereby unable to attain the bystander activity ascribed to MMAE and PBD ADCs.44 Bystander activity can be advantageous when tumor antigen expression is heterogeneous.44 However, the reduced uptake of cys-mcMMAF into nontargeted cells may mitigate toxicity, which could explain why SGN-CD19A shows reduced myelosuppression, minimal neuropathy, and improved overall tolerability when compared with other ADCs.12,45 SGN-CD19B, on the other hand, offers the potential for robust activity against refractory B-cell malignancies but may induce more myelosuppression than SGN-CD19A.12 We would not expect patients treated with SGN-CD19B to experience the ocular symptoms associated with certain auristatin and maytansine payloads46 because these reversible ocular events are caused by nonantigen mediated uptake of ADC and are not associated with the PBD payload.
Recent clinical studies in NHL have explored combining targeted therapies in order to improve clinical activity.47 Our previous work on SGN-CD19A revealed a synergistic mechanism between CD19 and CD20 whereby rituximab can influence the dynamics of CD19 surface expression leading to improved drug delivery of cys-mcMMAF.48 This prompted us to evaluate the activity of SGN-CD19B plus rituximab in vivo. The results confirmed favorable combination activity showing that SGN-CD19B induces durable regressions at one-third of the monotherapy dose when it is combined with rituximab. Similarly, preclinical experiments with INO revealed evidence of improved activity in combination with rituximab.49 Clinical trials employing INO plus rituximab showed impressive objective response rates of 87% for relapsed FL and 74% for relapsed DLBCL.50 In contrast, INO plus rituximab was less impressive in refractory aggressive NHL yielding a 20% objective response rate, possibly because of the fact that patients become refractory to rituximab in later lines of therapy. Mechanism of action studies suggest SGN-CD19B, like SGN-CD19A, shows improved internalization in the presence of rituximab (data not shown), likely resulting in increased drug delivery. Therefore, the ability to attain improved activity with SGN-CD19B plus rituximab may be dependent on whether this mechanism is retained in early as well as later lines of therapy.
B-NHL is a heterogeneous disease. A key molecular feature of aggressive B-cell lymphoma consists of genomic rearrangements that result in the overexpression of MYC, BCL2, or/and BCL6, referred to as double-hit or triple-hit lymphoma. Double-hit and triple-hit lymphomas are associated with a more aggressive disease course and poor survival because of the lack of effective treatment modalities.51,52 Our in vitro data showed that SGN-CD19B was widely active against the B-cell malignancies tested, including cell lines representing double-hit and triple-hit lymphoma. In vivo testing of SGN-CD19B also revealed compelling activity as 100% of mice bearing tumors established from the double-hit DLBCL cell line WSU-DLCL2 were cured of their tumors following treatment with 300 μg/kg of SGN-CD19B. The ABC cell-of-origin subtype of DLBCL has garnered much attention because of its association with substantially worse outcomes in response to standard chemotherapy.19 Because we were unable to establish xenograft models using the ABC DLBCL cell lines, we could not confirm the activity of SGN-CD19B against this subtype in vivo. However, we were able to demonstrate that ABC DLBCL cell lines are sensitive to SGN-CD19B in vitro (IC50 = 4-34 ng/mL) and that sensitivity is detectable even when cell lines showed less inherent sensitivity to PBD drug in pulsed studies with SGD-1882 (K.A.G., unpublished observations) or when cell lines had relatively low copy numbers of CD19 (see Figure 1, Ri-1 cell line). This suggests that SGN-CD19B could be active across different subtypes of aggressive lymphomas, including the ABC DLBCL subtype, provided they express CD19.
We have shown here that in addition to B-NHL, SGN-CD19B demonstrates impressive antitumor activity in B-ALL. In the NALM-6 disseminated model, 100% of mice treated with 100 μg/kg of SGN-CD19B were still alive out to 115 days, indicating they were likely cured of their disease. Even at the lowest dose of 10 μg/kg, 60% of the mice survived >115 days, whereas animals in the untreated and control groups survived <40 days. These data compare favorably to preclinical data shown for INO,53 which recently met its clinical end point in a pivotal phase 3 randomized trial in relapsed/refractory B-ALL demonstrating a significantly higher rate of complete response compared with standard chemotherapy (80.7% vs 29.4%, P < .001).54 The accessibility of leukemic blasts and confirmed antileukemic activity seen with DNA alkylating agents, including PBD ADCs,55 suggest that SGN-CD19B may have a high probability of technical success in B-ALL.
In summary, SGN-CD19B has therapeutic potential, delivering antitumor activity in B-NHL and B-ALL both in vitro and in vivo. SGN-CD19B releases a highly potent drug and works by a different mechanism of action compared with other ADC payloads such as auristatin-based ADCs suggesting it may offer distinct opportunities in the treatment of B-cell malignancies. A multicenter phase 1 dose escalation study is enrolling in order to further evaluate the therapeutic potential of SGN-CD19B in relapsed/refractory B-NHL (registered at www.clinicaltrials.gov as #NCT02702141).
Presented in part at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jamie Miyamoto and Kerry Klussman for performing in vitro cytotoxicity assays; Teresa Dominguez for help with quantitative FACS and cytotoxicity assays; Laurie Tatalick for support on nonclinical studies; and Ivan Stone, Martha Anderson, Weiping Zeng, and Michelle Urlich for conducting in vivo studies. The authors also thank Robert Lyon, Scott Jeffrey, and Steve Alley for critical reading of the manuscript.
Authorship
Contribution: M.C.R. designed experiments, interpreted data, prepared figures, and wrote the manuscript; M.C.P.-W. provided intellectual input and helped write the manuscript; H.K., C.Y., B.M., H.A.V.E., and K.A.G. designed and performed experiments, interpreted data, and prepared figures; B.S. designed experiments, interpreted data, and prepared figures; and D.B. provided guidance, intellectual input, and reviewed the manuscript.
Conflict-of-interest disclosure: All authors are current or former employees of Seattle Genetics, Inc. All studies were paid for by Seattle Genetics, Inc.
Correspondence: Maureen C. Ryan, Seattle Genetics, Inc., 21823 30th Dr SE, Bothell, WA 98021; e-mail: mryan@seagen.com.