Key Points
High doses of NK cells expanded ex vivo with mbIL21-expressing feeder cells can be safely infused posthaplotransplant.
Infusion of NK cells was associated with improved NK-cell function, low relapse, and incidence of viral infections.
Abstract
Relapse has emerged as the most important cause of treatment failure after allogeneic hematopoietic stem cell transplantation (HSCT). To test the hypothesis that natural killer (NK) cells can decrease the risk of leukemia relapse, we initiated a phase 1 dose-escalation study of membrane-bound interleukin 21 (mbIL21) expanded donor NK cells infused before and after haploidentical HSCT for high-risk myeloid malignancies. The goals were to determine the safety, feasibility, and maximum tolerated dose. Patients received a melphalan-based reduced-intensity conditioning regimen and posttransplant cyclophosphamide-based graft-versus-host disease (GVHD) prophylaxis. NK cells were infused on days −2, +7, and +28 posttransplant. All NK expansions achieved the required cell number, and 11 of 13 patients enrolled received all 3 planned NK-cell doses (1 × 105/kg to 1 × 108/kg per dose). No infusional reactions or dose-limiting toxicities occurred. All patients engrafted with donor cells. Seven patients (54%) developed grade 1-2 acute GVHD (aGVHD), none developed grade 3-4 aGVHD or chronic GVHD, and a low incidence of viral complications was observed. One patient died of nonrelapse mortality; 1 patient relapsed. All others were alive and in remission at last follow-up (median, 14.7 months). NK-cell reconstitution was quantitatively, phenotypically, and functionally superior compared with a similar group of patients not receiving NK cells. In conclusion, this trial demonstrated production feasibility and safety of infusing high doses of ex vivo–expanded NK cells after haploidentical HSCT without adverse effects, increased GVHD, or higher mortality, and was associated with significantly improved NK-cell number and function, lower viral infections, and low relapse rate posttransplant.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is effective treatment of patients with advanced hematological malignancies.1 After progressive improvements in treatment-related mortality,2 disease relapse emerged as the most important cause of treatment failure.3 Hence there is urgent need for novel therapies to reduce the risk of relapse posttransplant.
Natural killer (NK) cells have the capability to eliminate leukemic or virally infected cells.4,5 In mice, NK cells have been shown to improve engraftment and decrease graft-versus-host disease (GVHD) after transplantation.6,7 Higher absolute NK-cell numbers in the early posttransplant period were associated with lower relapse and improved survival.8,9 Moreover, NK-cell alloreactivity was reported to decrease relapse rate after haploidentical transplantation (haploHSCT).10
Several studies have used NK cells from the peripheral blood (PB) of the donor collected by steady-state apheresis, with typical doses ranging from 1 × 107/kg to 3 × 107/kg.11-15 Most studies showed no major toxicities, except in 1 report, in which infusion of interleukin 15 (IL-15)/4-1BBL–activated NK cells was associated with a high incidence of acute GVHD (aGVHD).16
Obtaining sufficient numbers of NK cells to achieve a therapeutic effect has been a major limitation.17 Attempts to expand NK cells have typically used IL-2 and/or IL-15.18-24 Our group developed a method to expand NK cells ex vivo using K562 feeder cells expressing membrane-bound IL-21 (mbIL21).25 This approach expands NK cells up to 35 000-fold in 3 weeks and produces highly functional NK cells.25
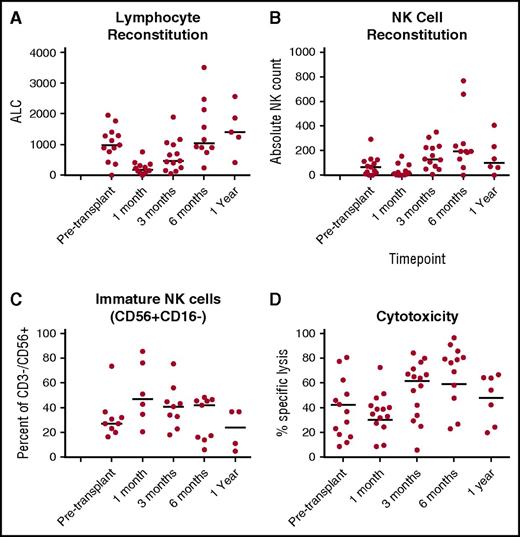
NK cells are the first cells to recover after transplant; however, their function is significantly impaired.26-28 We also observed that absolute NK-cell numbers were low in the first month following T-cell replete haploHSCT with posttransplant cyclophosphamide, and had immature phenotype and markedly decreased function (Figure 1).29 Therefore, we hypothesized that multiple infusions of high numbers of mature, fully functional mbIL21-expanded NK cells before and after transplantation would improve antitumor activity for high-risk myeloid malignancies. We performed a phase 1 study to determine safety, feasibility, and maximum tolerated dose (MTD) of this approach.
NK-cell number, phenotype and function in the first year posttransplant for patients treated with haploidentical stem cell transplantation using posttransplant cyclophosphamide on protocol 2009-0266 (without NK-cell infusions). (A) Absolute lymphocyte count (ALC) was determined from a clinical complete blood count obtained at the indicated time point. (B) Absolute NK-cell counts were determined from PB samples obtained at same time points, from which PBMCs were isolated and cryopreserved for batch testing. CD3−CD56+ populations were determined from within lymphocyte gates, and absolute NK count derived according to the percent of CD3−CD56+ cells. (C) NK-cell maturity was determined according to CD16+ and CD16− fractions of the NK cells in Figure 3B. (D) NK-cell function at 1 month posttransplant was determined by measuring cytotoxicity against 721.221 targets, wherein PBMCs were applied according to NK-cell content at a 40:1 NK-to-target ratio.
NK-cell number, phenotype and function in the first year posttransplant for patients treated with haploidentical stem cell transplantation using posttransplant cyclophosphamide on protocol 2009-0266 (without NK-cell infusions). (A) Absolute lymphocyte count (ALC) was determined from a clinical complete blood count obtained at the indicated time point. (B) Absolute NK-cell counts were determined from PB samples obtained at same time points, from which PBMCs were isolated and cryopreserved for batch testing. CD3−CD56+ populations were determined from within lymphocyte gates, and absolute NK count derived according to the percent of CD3−CD56+ cells. (C) NK-cell maturity was determined according to CD16+ and CD16− fractions of the NK cells in Figure 3B. (D) NK-cell function at 1 month posttransplant was determined by measuring cytotoxicity against 721.221 targets, wherein PBMCs were applied according to NK-cell content at a 40:1 NK-to-target ratio.
Methods
Patients
Patients 18 to 65 years of age with high-risk acute myeloid leukemia (AML), myelodysplastic syndromes (MDSs), or chronic myeloid leukemia (CML) (≤5% bone marrow blasts), adequate performance status, and organ function were included. High-risk myeloid malignancies were assessed for inclusion as follows:
AML with high-risk disease by refractoriness to induction chemotherapy, cytogenetics, and/or molecular mutations, in morphologic remission (≤5% bone marrow blasts),
MDS with intermediate- or high-risk International Prognostic Scoring System (IPPS) score, or
CML that failed treatment with tyrosine kinase inhibitors or progressed to accelerated or blast phase, with ≤5% bone marrow blasts.
Patients included were 18 to 65 years of age with adequate performance status (≥70% Karnofsky) and organ function (ejection fraction, ≥40%; corrected diffusing capacity of the lungs for carbon monoxide, ≥50%; Br, <1.5 mg/dL; alanine transaminase/aspartate transaminase, <200 IU/mL; creatinine clearance by Cockroft-Gault, ≥50 mL per minute). Exclusion criteria included: active hepatitis B, hepatitis C, or HIV-positive, liver cirrhosis, uncontrolled infections, central nervous system involvement for <3 months, and positive pregnancy test in a woman with childbearing potential.
Donors
Donors were required to be at least 16 years of age and must have agreed to 2 donations: ∼1 unit (500 mL of peripheral blood) collected on day −16 for NK-cell production and bone marrow progenitor cells obtained via a bone marrow harvest procedure with the goal of 3 × 108 total nucleated cell dose (TNC) per kilogram collected and infused on day 0 of transplant.
Transplant conditioning regimen and stem cell infusion
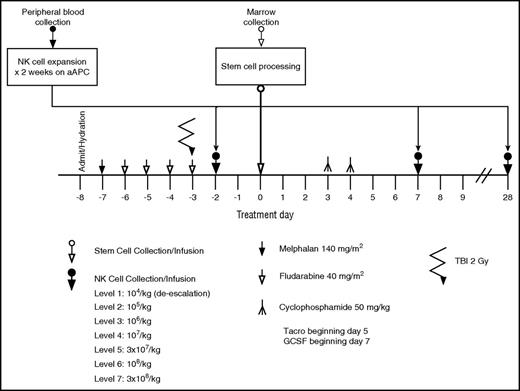
All patients were treated with a conditioning regimen consisting of melphalan 140 mg/m2 for 1 dose on day −7, fludarabine 160 mg/m2 divided in 4 daily doses on days −7 to −4. Older patients (>55 years) or those with comorbidities could receive reduced doses of melphalan at 100 mg/m2 at the discretion of the treating physician, as we previously described. All patients received 1 dose of 200 cGy total-body irradiation on day −3 (Figure 2). A bone marrow graft was infused fresh on day 0. The goal of the bone marrow harvest collection was 3 × 108 TNC per kilogram. GVHD prophylaxis consisted of posttransplant cyclophosphamide at 50 mg/kg per day on days +3 and +4, tacrolimus starting on day +5 and continued for 6 months in the absence of GVHD, and mycophenolate mofetil starting on day +5 and continued for 3 months, unless otherwise indicated. All patients received filgrastim at 5 μg/kg per day starting on day +7 until the absolute neutrophil count (ANC) reached 1500 × 109/L. Standard antimicrobial prophylaxis was provided with voriconazole, pentamidine or trimethoprim-sulfamethoxazole, and acyclovir or valacyclovir for fungal, Pneumocystis jiroveci, and herpes simplex, respectively.
Treatment schema for the clinical trial 2012-0708 of adding infusions of expanded donor NK cells to haploidentical stem cell transplant with posttransplant cyclophosphamide.
Treatment schema for the clinical trial 2012-0708 of adding infusions of expanded donor NK cells to haploidentical stem cell transplant with posttransplant cyclophosphamide.
Ex vivo–expanded NK-cell product
The leukocyte fraction (“buffy coat”) of haploidentical blood collected on day −16 was the source of PB mononuclear cells (PBMCs). T cells were magnetically separated with colloidal superparamagnetic anti-CD3 monoclonal antibody (mAb; Miltenyi Biotec, Auburn, CA). NK cells from the transplant donor were expanded ex vivo for 14 days from CD3-depleted PBMCs (see supplemental Methods, available on the Blood Web site) by adding irradiated K562 Clone9.mbIL21 feeder cells once weekly, as previously described25 (hereafter denoted as mbIL21-NK cells). Fresh mbIL21-NK cells were infused on day −2. The remaining mbIL21-NK cells were cryopreserved (40% Plasmalyte, 50% human AB serum, 10% dimethyl sulfoxide) and were thawed, washed, and infused on days +7 and +28 (or later, up to day +90) (Figure 2). The cryopreserved NK cells were prepared for infusion by thawing in a 37°C water bath, washing once with infusion buffer (0.5% human serum albumin in Plasmalyte A) and resuspending the final cell dose in 100 mL of infusion buffer. Dose escalation was planned in cohorts of a minimum of 2 patients starting at 1 × 105/kg per dose and escalating by one-half-log increments up to 3 × 108/kg per dose or until the MTD was reached. A “safe dose” level of 1 × 104/kg per dose was used if a patient needed treatment before the 1 × 105/kg per dose level was deemed safe.
HLA typing and determination of KIR content/matching
Killer cell immunoglobulin-like receptor (KIR) genotyping and alloreactivity were evaluated. NK alloreactivity in the graft-versus-host direction and/or KIR B genotype was preferred for donor selection, but not required.30,31 KIR genotyping and alloreactivity were evaluated. NK alloreactivity in the GVHD direction and/or KIR B genotype was preferred for donor selection, but not required. All patients had HLA typing by intermediate- and/or high-resolution typing. KIR genotyping was performed for selected NK-cell donors with the reverse sequence-specific oligonucleotide method utilizing fluorescently labeled beads conjugated to oligonucleotide probes and detected in a Luminex platform (One Lambda, Canoga Park, CA). KIR ligand-ligand mismatch was predicted using KIR Ligand Calculator (http://www.ebi.ac.uk/ipd/kir/ligand.html). KIR receptor-ligand mismatch was refined by ligand-ligand mismatch prediction based on presence of the KIR receptor gene in the donor. KIR-B content was determined using B Content Calculator (http://www.ebi.ac.uk/ipd/kir/donor_b_content.html).
NK-cell phenotype and function
PBMCs from patients and aliquots of donor mbIL21-NK cells were cryopreserved for batched testing of function and phenotype. PB was obtained around day +30 from patients receiving haploHSCT without NK cells (The University of Texas MD Anderson Cancer Center [MDACC] #2009-0266, clinicaltrials.gov NCT01010217; control group), and prior to the third infusion of NK cells around day +28 from patients receiving haploHSCT with NK cells (MDACC #2012-0708, clinicaltrials.gov NCT01904136), both referred to hereafter as day +28 samples. PBMCs from patients and aliquots of donor NK-cell infusion products were cryopreserved for batched testing of function and phenotype. Cells were thawed and rested overnight in 100 IU/mL IL-2 prior to testing. The calcein-release assay was used to determine cytotoxicity as previously described.32 Cytokine production was determined by stimulating effectors (adjusted for NK-cell content) with targets at a 2:1 NK-to-target ratio for 3 hours in the presence of Golgi-stop (BD Biosciences) and metal-conjugated anti-CD107a, followed by labeling with 5 μM cisplatin and surface and intracellular staining with metal-conjugated antibodies. NK cells were identified as CD56+CD3− and data were acquired by CyTOF (DVS Sciences) as previously described.33 The antibody clones and heavy metal labels are listed in supplemental Table 2.
Analysis of mass cytometry data
To unbias differences in sample event number between samples, an equal number of events from each CD3−CD56+ gated sample were exported using FlowJo. Clustering analysis was then performed using spanning-tree progression analysis of density-normalized events (SPADE V3.0) on CytoBank, downsampled to 3000 events per sample, and using the following markers for clustering: CD3, CD4, CD8a, CD11a, CD16, CD56, CD57, CD62L, CD94, CD107a, CD134 (OX40), CD223 (LAG3), CD226 (DNAM-1), CD253 (TRAIL), CD272 (BTLA), CD314 (NKG2D), CD357 (GITR), NKp30, NKp44, NKp46, NKp80, NKG2A, NKG2C, and TIM3. For ViSNE (visual interactive stochastic neighbor embedding) analysis, the equalized samples were then concatenated for each group (PB from patients on 2009-0266 and 2012-0708, and NK-cell infusion products), and an equal number of events per group was then exported for clustering in order to weight each group equally. ViSNE analysis of group data were then performed on CytoBank, using the same markers for clustering as for SPADE analysis.
For unsupervised clustering of mass cytometry data, rows were centered, and unit variance scaling was applied to rows. Both rows and columns were clustered using correlation distance and average linkage and ordered by tightest clusters first. For principal components analysis, unit variance scaling was applied to rows and singular value decomposition with imputation was used to calculate principal components. Prediction ellipses were generated such that with probability 0.95, a new observation from the same group will fall inside the ellipse. Principal components analysis, heatmaps, and clustering were generated using the ClustVis webtool (http://biit.cs.ut.ee/clustvis/).
Trial design
The primary objectives were to evaluate the safety of infusion and determine the MTD of 3 doses of haploidentical mbIL21-NK cells administered in conjunction with haploHSCT (days −2, +7, and +28-90 posttransplant). Secondary objectives were to determine NK-cell persistence after infusion, immunologic reconstitution, phenotype, and function during immune reconstitution, estimate the proportion of patients with engraftment/graft failure, 100-day nonrelapse mortality (NRM), cumulative incidence (CI) of grade 2-4 aGVHD and chronic GVHD (cGVHD), relapse rate (RR), overall survival (OS), and disease-free survival (DFS). These measures were compared with a control group.
The trial was approved by the institutional review board of MDACC and conducted under an investigational new drug application from the US Food and Drug Administration. All patients and donors provided written informed consent according to the Declaration of Helsinki.
For the purpose of dose finding, “toxicity” was defined as any of the events (1) death, (2) grade 3-4 infusion reaction, (3) grade 4 organ toxicity (not including mucositis or myelosuppression), (4) graft failure, or (5) severe (grade 3 or 4) GVHD occurring within 72 days of the first NK-cell infusion on day −2 or day −1, that is, by day +70 post-SCT. Consequently, the time window for evaluating toxicity was 72 days, from the day of the first NK-cell infusion to day +70. Patient outcome was from the time of occurrence of toxicity or, if toxicity has not yet occurred by an observation time prior to day +70 posttransplant, the outcome will be the patient’s follow-up time without toxicity. Denoting the time of follow-up or toxicity by T, and the right-censoring indicator C = 1 if T was a follow-up time without toxicity and C = 0 if T was the time of observed toxicity, each patient’s data consisted of the pair (T,C), with T no larger than 72 days. A patient’s outcome (T,C) will be considered “fully evaluated” if either C = 0 (toxicity has occurred at some time up to day 70 posttransplant) or (T,C) = (72,1), which says that no toxicity has occurred by day +70.
Dose finding was carried out using the time-to-event (TiTE) continual reassessment method (CRM) of Cheung and Chappell. Denoting exponentiation by “E,” the 7 NK-cell per-administration doses (PADs) to be studied are number of NK cells per kilogram = 105, 106, 107, 3 × 107, 108, and 3 × 108. The TiTE CRM was applied with cohorts of size 2, starting at 105 (the second lowest NK-cell PAD level), not skipping a dose level when escalating, target Pr (toxicity by day 70 posttransplant) = 0.50, and maximum sample size of 30. To avoid aggressive/unsafe escalation early in the trial, the design was to proceed in 2 stages. In stage 1, the minimum waiting time between dose cohorts is 49 days from the first NK infusion. For mb21-NK-cell infusion, the patient must have been off systemic corticosteroids for ≥72 hours (<0.5 mg/kg prednisone), have no active grade II-IV aGVHD, no uncontrolled infections or fever (>38.5°C), and for third infusion, no grade ≥3 nonhematologic organ toxicity. The first cohort of 2 patients will be enrolled at dose = 105 NK cells. If no toxicity is observed, then 2 new patients (the second cohort) will be enrolled at dose = 106 NK cells. If no toxicities are observed among the previously accrued patients, then escalation will proceed in cohorts of size 2 to subsequently higher doses. If a new patient arrives to be treated at a time when either 1 or 2 of the patients in the previous cohort have been treated but not yet fully evaluated, then the new patient will be treated at 1 dose level below the current recommended level, up to a maximum of 3 new patients. Stage 2 begins when the first toxicity occurs, and thereafter the cohort size will be 1 with the TiTE-CRM used to choose each new patient’s dose, using the fixed target 0.50 for Pr (toxicity within 72 days | dose). The “optimal” dose was defined as that for which the posterior mean of Pr (toxicity within 72 days | dose) given the current data are closest to 0.50. Each subject will be followed for the entire 72-day period for toxicity evaluation. Once a dose-limiting toxicity (DLT) occurs at any time in the 72-day window for any subject, new subjects will be assigned to the optimal dose. The following additional safety restrictions were applied during the trial: (1) an untried dose may not be skipped when escalating and (2) escalation may not be done immediately after a toxic outcome (ie, incoherent escalation). The skeleton corresponding to dose levels 1 to 7, that is the basis for the assumed TiTE CRM model, is (0.30, 0.35, 0.42, 0.50, 0.60, 0.68, 0.75).
Description of retrospective control group
The control group of patients (n = 45;median age, 45 years) with AML/MDS/CML in first or second complete remission (CR1/CR2) or morphologic complete remission (CR) (≤5% bone marrow blasts) was treated on a previous clinical trial with the same conditioning regimen but without NK cells (MDACC protocol 2009-0266) (supplemental Table 2). Patient characteristics were similar with those of the mb21-NK-cell treated group (supplemental Table 1). All patients achieved primary engraftment, with median time to neutrophil and platelet engraftment of 18 and 40 days, respectively. Grade 2-4 aGVHD occurred in 14 of 44 patients (31.8%), grade 3-4 aGVHD in 1 of 41 patients (2.3%), and cGVHD occurred in 7 of 41 patients (17%). Nine patients (20%) died of treatment-related mortality (TRM), 10 patients (22%) relapsed, and 25 patients (55.5%) were alive at last follow-up (Figure 3A-E).
Comparison of clinical outcomes between patients treated on protocols 2012-0708 (with NK cells [blue line]) and 2009-0266 (no NK cells [red line]). Data from 2009-0266 were censored to include patients with only AML/MDS and CML in morphologic CR at the time of transplant. (A) Time to neutrophil engraftment. (B) Time to platelet engraftment. (C) Cumulative incidence of grade 2-4 acute GVHD. (D) Cumulative incidence of grade 3-4 aGVHD. (E) Cumulative incidence of chronic GVHD limited + extensive. (F) Incidence of BKV cystitis. (G) Incidence of CMV reactivation. (H) Cumulative incidence of relapse. (I) Probability of progression-free survival. Kaplan-Meier survival curves were compared using the log-rank method. BKV, BK polyomavirus.
Comparison of clinical outcomes between patients treated on protocols 2012-0708 (with NK cells [blue line]) and 2009-0266 (no NK cells [red line]). Data from 2009-0266 were censored to include patients with only AML/MDS and CML in morphologic CR at the time of transplant. (A) Time to neutrophil engraftment. (B) Time to platelet engraftment. (C) Cumulative incidence of grade 2-4 acute GVHD. (D) Cumulative incidence of grade 3-4 aGVHD. (E) Cumulative incidence of chronic GVHD limited + extensive. (F) Incidence of BKV cystitis. (G) Incidence of CMV reactivation. (H) Cumulative incidence of relapse. (I) Probability of progression-free survival. Kaplan-Meier survival curves were compared using the log-rank method. BKV, BK polyomavirus.
Results
Patients and transplant characteristics
Thirteen patients (6 men, 7 women; median age, 44 years; range, 18-60 years) were enrolled and treated on this phase 1 clinical trial between May 2014 and January 2016. The majority of patients had a high risk of disease relapse (Table 1).
Characteristics and clinical outcomes of patients treated on the phase 1 clinical trial
| Patient . | Age, y . | Sex . | Diagnosis . | Disease characteristics . | Disease status at SCT . | MRD . | Relapse risk . | Donor . | Engraftment . | Time to ANC 500 . | Chimerism at day 28 . | Grade 2-4 aGVHD . | Grade 3-4 aGVHD . | Systemic steroids . | cGVHD . | Outcome of SCT . | Last follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | CML | Refractory to TKIs | CP1 | − | Int | Son | Yes | 20 | Mixed | No | No | No | No | Died (TRM) | Day +85 |
| 2 | 52 | F | AML | FLT3+ | Relapse pretransplant | + | High | Son | Yes | 17 | Donor | Yes | No | Yes | No | Relapsed | Day +582 |
| 3 | 60 | M | CML, MDS | Monosomy 7 | CP1 | − | High | Daughter | Yes | 27 | Donor | Yes | No | No | No | Remission | Day +755 |
| 4 | 38 | F | AML | PIF, cx. cyto, MLL+ | CR1 | + | High | Sister | Yes | 16 | Donor | No | No | No | No | Remission | Day +410 |
| 5 | 43 | M | AML | PIF, diploid cyto | CR1 | − | High | Brother | Yes | 18 | Donor | Yes | No | Yes | No | Remission | Day +419 |
| 6 | 34 | M | CML | BP CNS+ | CP2 | − | High | Sister | Yes | 21 | Donor | No | No | No | No | Remission | Day +481 |
| 7 | 23 | M | AML | FLT3+ | CR1 | − | High | Sister | Yes | 15 | Donor | No | No | No | No | Remission | Day +417 |
| 8 | 18 | F | AML | ASXL1+ | CR1 | + | High | Father | Yes | 21 | Donor | Yes | No | Yes | No | Remission | Day +316 |
| 9 | 58 | F | AML | MLL+ | CR1 | − | High | Daughter | Yes | 19 | Donor | Yes | No | No | No | Remission | Day +412 |
| 10 | 45 | F | CML | BP CNS+ | CP2 | − | High | Brother | Yes | 19 | Donor | Yes | No | Yes | No | Remission | Day +477 |
| 11 | 55 | M | AML | MLL+ | CR1 | + | High | Son | Yes | 14 | Donor | No | No | No | No | Remission | Day +448 |
| 12 | 25 | F | AML | PIF, diploid cyto | CR1 | − | High | Sister | Yes | 17 | Donor | Yes | No | Yes | No | Remission | Day +363 |
| 13 | 48 | F | CML | Refractory to TKIs | CP1 | + | Int | Daughter | Yes | 19 | Donor | No | No | No | No | Remission | Day +236 |
| Patient . | Age, y . | Sex . | Diagnosis . | Disease characteristics . | Disease status at SCT . | MRD . | Relapse risk . | Donor . | Engraftment . | Time to ANC 500 . | Chimerism at day 28 . | Grade 2-4 aGVHD . | Grade 3-4 aGVHD . | Systemic steroids . | cGVHD . | Outcome of SCT . | Last follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | CML | Refractory to TKIs | CP1 | − | Int | Son | Yes | 20 | Mixed | No | No | No | No | Died (TRM) | Day +85 |
| 2 | 52 | F | AML | FLT3+ | Relapse pretransplant | + | High | Son | Yes | 17 | Donor | Yes | No | Yes | No | Relapsed | Day +582 |
| 3 | 60 | M | CML, MDS | Monosomy 7 | CP1 | − | High | Daughter | Yes | 27 | Donor | Yes | No | No | No | Remission | Day +755 |
| 4 | 38 | F | AML | PIF, cx. cyto, MLL+ | CR1 | + | High | Sister | Yes | 16 | Donor | No | No | No | No | Remission | Day +410 |
| 5 | 43 | M | AML | PIF, diploid cyto | CR1 | − | High | Brother | Yes | 18 | Donor | Yes | No | Yes | No | Remission | Day +419 |
| 6 | 34 | M | CML | BP CNS+ | CP2 | − | High | Sister | Yes | 21 | Donor | No | No | No | No | Remission | Day +481 |
| 7 | 23 | M | AML | FLT3+ | CR1 | − | High | Sister | Yes | 15 | Donor | No | No | No | No | Remission | Day +417 |
| 8 | 18 | F | AML | ASXL1+ | CR1 | + | High | Father | Yes | 21 | Donor | Yes | No | Yes | No | Remission | Day +316 |
| 9 | 58 | F | AML | MLL+ | CR1 | − | High | Daughter | Yes | 19 | Donor | Yes | No | No | No | Remission | Day +412 |
| 10 | 45 | F | CML | BP CNS+ | CP2 | − | High | Brother | Yes | 19 | Donor | Yes | No | Yes | No | Remission | Day +477 |
| 11 | 55 | M | AML | MLL+ | CR1 | + | High | Son | Yes | 14 | Donor | No | No | No | No | Remission | Day +448 |
| 12 | 25 | F | AML | PIF, diploid cyto | CR1 | − | High | Sister | Yes | 17 | Donor | Yes | No | Yes | No | Remission | Day +363 |
| 13 | 48 | F | CML | Refractory to TKIs | CP1 | + | Int | Daughter | Yes | 19 | Donor | No | No | No | No | Remission | Day +236 |
AML, acute myeloid leukemia; ANC, absolute neutrophil count; BP, blast phase; CML, chronic myeloid leukemia; CNS, central nervous system; CP1, chronic phase 1; CP2, chronic phase 2; CR1, first complete remission; CR2, second complete remission; cx., complex; cyto, complex cytogenetics; F, female; FLT3, FMS-like tyrosine kinase 3; GVHD, graft-versus-host disease; Int, intermediate; M, male; MDS, myelodysplastic syndrome; MLL, mixed lineage leukemia gene; MRD, minimal residual disease; PIF, primary induction failure; SCT, stem cell transplant; TKI, tyrosine kinase inhibitor.
Characteristics of the mbIL21-NK cells, toxicities, and NK-cell reconstitution posttransplant
mbIL21-NK-cell characteristics and infusional toxicities.
Median purity of the product was 98.98% (CD3−CD16/56+) with median viability of 97% and extremely low or undetectable T- and B-cell content (Table 2). Donor and recipient KIR profiles are presented in Table 3. Five patients had KIR mismatch with the donor based on the ligand-ligand model, 4 donors had high B content, 4 had “better,” 1 “best,” and 8 “neutral” KIR score; 4 patients had a KIR2DS1 donor. Five patients had donors without any NK-cell good-prognosis predictors.
Characteristics of the mbIL21-NK-cell product
| Patient . | Viability, % . | Viable CD32+ cells, . | CD3+ cells, . | CD3−CD(16,56)+cells, . | CD19+ cells, . | CD14+ cells, . |
|---|---|---|---|---|---|---|
| APCs, % . | T cells: %, ×105/kg . | NK cells, % . | B cells, % . | monocytes, % . | ||
| 1 | 97 | 0.1 | 0.02, 0.00002 | 98.31 | Not detected | Not detected |
| 2 | 98 | 0.35 | 0.35, 0.0035 | 98.01 | Not detected | Not detected |
| 3 | 96 | 0.5 | 0.01, 0.0001 | 99.38 | Not detected | Not detected |
| 4 | 97 | 0.1 | 0.01, 0.001 | 98.77 | Not detected | Not detected |
| 5 | 98 | 0.38 | Not detected | 96.73 | Not detected | 0.11 |
| 6 | 96 | 0.07 | 0.06, 0.006 | 98.95 | Not detected | Not detected |
| 7 | 97 | 0.15 | Not detected | 99.46 | Not detected | 0.05 |
| 8 | 98 | 0.1 | 0.04, 0.04 | 98.46 | Not detected | Not detected |
| 9 | 98 | 0.24 | 0.03, 0.03 | 99.81 | Not detected | Not detected |
| 10 | 96 | 0.31 | 0.02, 0.06 | 99.80 | Not detected | Not detected |
| 11 | 97 | Not detected | 0.01, 0.03 | 99.72 | Not detected | Not detected |
| 12 | 97 | 0.07 | 0.07, 0.7 | 98.98 | Not detected | Not detected |
| 13 | 93 | 0.33 | 0.03, 0.3 | 99.33 | 0.54 | Not detected |
| Patient . | Viability, % . | Viable CD32+ cells, . | CD3+ cells, . | CD3−CD(16,56)+cells, . | CD19+ cells, . | CD14+ cells, . |
|---|---|---|---|---|---|---|
| APCs, % . | T cells: %, ×105/kg . | NK cells, % . | B cells, % . | monocytes, % . | ||
| 1 | 97 | 0.1 | 0.02, 0.00002 | 98.31 | Not detected | Not detected |
| 2 | 98 | 0.35 | 0.35, 0.0035 | 98.01 | Not detected | Not detected |
| 3 | 96 | 0.5 | 0.01, 0.0001 | 99.38 | Not detected | Not detected |
| 4 | 97 | 0.1 | 0.01, 0.001 | 98.77 | Not detected | Not detected |
| 5 | 98 | 0.38 | Not detected | 96.73 | Not detected | 0.11 |
| 6 | 96 | 0.07 | 0.06, 0.006 | 98.95 | Not detected | Not detected |
| 7 | 97 | 0.15 | Not detected | 99.46 | Not detected | 0.05 |
| 8 | 98 | 0.1 | 0.04, 0.04 | 98.46 | Not detected | Not detected |
| 9 | 98 | 0.24 | 0.03, 0.03 | 99.81 | Not detected | Not detected |
| 10 | 96 | 0.31 | 0.02, 0.06 | 99.80 | Not detected | Not detected |
| 11 | 97 | Not detected | 0.01, 0.03 | 99.72 | Not detected | Not detected |
| 12 | 97 | 0.07 | 0.07, 0.7 | 98.98 | Not detected | Not detected |
| 13 | 93 | 0.33 | 0.03, 0.3 | 99.33 | 0.54 | Not detected |
Characteristics of the NK-cell doses, donor/recipient KIR characteristics, and infusional toxicities
| Patient . | NK cells per dose, /kg . | No. of doses . | Patient KIR ligand . | Donor KIR ligand . | NK alloreactivity . | Donor KIR haplotype . | No. of Cen B/B . | KIR score . | KIR centromeric . | KIR 2DS1 . | Toxicity . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 × 104 | 2 | C2/C2, BW4 | C2, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 2 | 1 × 105 | 3 | C1/C2, Bw4 | C1, Bw4 | No | A/B | 2 | Better | Cen-A/B | No | None |
| 3 | 1 × 105 | 3 | C1/C2, Bw4 | C1/C2, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 4 | 1 × 106 | 3 | C1/C1, Bw4 | C1/C2, Bw4 | Yes | A/B | 2 | Better | Cen-A/B | No | None |
| 5 | 1 × 106 | 3 | C1/C1 | C1/C2, Bw4 | Yes | A/A | 0 | Neutral | Cen-A/A | No | None |
| 6 | 1 × 106 | 2 | C1/C2, Bw4 | C1/C2, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 7 | 1 × 107 | 3 | C1/C2, Bw4 | C1, Bw4 | No | A/B | 2 | Best | Cen-B/B | Yes | None |
| 8 | 1 × 107 | 3 | C1/C1, Bw4 | C1, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 9 | 1 × 107 | 3 | C1/C1, Bw4 | C1/C1, Bw4 | No | A/B | 0 | Neutral | Cen-A/B | Yes | None |
| 10 | 3 × 107 | 3 | C1/C1 | C1/C2 | Yes | A/B | 2 | Better | Cen-A, Cen/Tel-B | No | None |
| 11 | 3 × 107 | 3 | C2/C2, Bw4 | C1/C2, Bw4 | Yes | A/B | 2 | Better | Cen-A/B | Yes | None |
| 12 | 1 × 108 | 3 | C1/C1, Bw4 | C1/C2, Bw4 | Yes | B/B | 0 | Neutral | Cen/Tel-B | Yes | None |
| 13 | 1 × 108 | 3 | C1/C1, Bw6 | C1/C1, Bw6 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| Patient . | NK cells per dose, /kg . | No. of doses . | Patient KIR ligand . | Donor KIR ligand . | NK alloreactivity . | Donor KIR haplotype . | No. of Cen B/B . | KIR score . | KIR centromeric . | KIR 2DS1 . | Toxicity . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 × 104 | 2 | C2/C2, BW4 | C2, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 2 | 1 × 105 | 3 | C1/C2, Bw4 | C1, Bw4 | No | A/B | 2 | Better | Cen-A/B | No | None |
| 3 | 1 × 105 | 3 | C1/C2, Bw4 | C1/C2, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 4 | 1 × 106 | 3 | C1/C1, Bw4 | C1/C2, Bw4 | Yes | A/B | 2 | Better | Cen-A/B | No | None |
| 5 | 1 × 106 | 3 | C1/C1 | C1/C2, Bw4 | Yes | A/A | 0 | Neutral | Cen-A/A | No | None |
| 6 | 1 × 106 | 2 | C1/C2, Bw4 | C1/C2, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 7 | 1 × 107 | 3 | C1/C2, Bw4 | C1, Bw4 | No | A/B | 2 | Best | Cen-B/B | Yes | None |
| 8 | 1 × 107 | 3 | C1/C1, Bw4 | C1, Bw4 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
| 9 | 1 × 107 | 3 | C1/C1, Bw4 | C1/C1, Bw4 | No | A/B | 0 | Neutral | Cen-A/B | Yes | None |
| 10 | 3 × 107 | 3 | C1/C1 | C1/C2 | Yes | A/B | 2 | Better | Cen-A, Cen/Tel-B | No | None |
| 11 | 3 × 107 | 3 | C2/C2, Bw4 | C1/C2, Bw4 | Yes | A/B | 2 | Better | Cen-A/B | Yes | None |
| 12 | 1 × 108 | 3 | C1/C1, Bw4 | C1/C2, Bw4 | Yes | B/B | 0 | Neutral | Cen/Tel-B | Yes | None |
| 13 | 1 × 108 | 3 | C1/C1, Bw6 | C1/C1, Bw6 | No | A/A | 0 | Neutral | Cen-A/A | No | None |
Cen, centromere.
NK-cell expansion met release criteria and dose requirements for all patients at all dose levels. All but 2 patients received all 3 planned mbIL21-NK-cell infusions (Table 3). The patient treated at the −1 dose level (1 × 104/kg per dose) developed secondary graft failure related to infection, and died of NRM unrelated to the NK-cell infusion.
NK-cell reconstitution posttransplant.
We assessed quantitative, phenotypic, and functional NK reconstitution at day +28 (prior to the third NK-cell infusion) using in vitro cytotoxicity assays and mass cytometry, and compared this with NK-cell reconstitution from similar patients in the control group and with the mbIL21-NK-cell infusion product.32
Compared with a control group, NK-cell activity in PBMCs from patients treated with NK cells was significantly greater (Figure 4A). Although degranulation (measured by CD107a) was similar (Figure 4B), patients who received NK cells also had greater proportion of NK cells that secreted TNF-α and IFN-γ in response to 721.221 targets (Figure 4B). CD62L cleavage from the NK-cell surface was greater for patients who received NK-cell infusions (Figure 4C), suggesting that increased activation in response to targets, and a functional shift from cytokine production to cytotoxicity, was significantly associated with increasing NK-cell dose (Figure 4C).
Assessment of phenotype and function of mbIL21-expanded NK-cell infusion product (green) or PB NK cells patients. Mononuclear cells were isolated from PBMCs of patients on protocol 2009-0266 (without NK cells [red]) or 2012-0708 (with NK cells [green]) ∼28 days after stem cell transplant. Samples from patients on protocol 2012-0708 were obtained prior to receiving the third dose of NK cells (∼3 weeks after receiving the second dose). (A) Cytotoxicity against 721.221 targets. Cells were applied to the cytotoxicity assay at a 10:1 NK-to-target ratio (according to NK-cell content determined by immune phenotyping). (B) NK-cell responses to stimulation with 721.221 targets at a 2:1 NK-to-target ratio for 3 hours. Degranulation (CD107a), cytokine production, and CD62L cleavage were determined by mass cytometry. (C) Cytotoxicity and IFNγ production from panels A and B stratified according to cell dose received (low, ≤106/kg per dose; high, ≥107/kg per dose). P values shown for unpaired Student t test. (D) Differences in phenotype of NK cells at day +28 posttransplant as determined by mass cytometry. Shown are surface markers on NK cells from supplemental Figure 1 that were significantly different between 2009-0266 (no NK-cell infusions) and 2012-0708 (with NK-cell infusions) using multiple unpaired Student t-test comparisons followed by the 2-stage false discovery approach. Corrected P values are indicated as *P < .01, **P < .001, ***P < .0001, or ****P < .00001. (E) Heatmap with unsupervised clustering analysis of the NK-cell phenotypes from supplemental Figure 1 according to relative expression of each receptor in each sample. Sample origin is indicated along the top row: day +28 PB-NK samples from protocol 2009-0266 (red) or 2012-0708 (blue), or NK-cell product (green). (F) SPADE trees of NK-cell subsets present in the NK-cell product or peripheral blood at day +28 posttransplant. Given the small number of samples in each group, KIR expression was excluded from clustering to avoid bias from individual KIR and HLA genotypes. (G) Heatmap with unsupervised clustering analysis of the NK-cell subsets from each sample according to those identified in panel F (using only nodes constituting at least 1% of any 1 sample). Sample origin color coding is as in panel E. (H) Principal components analysis based on the percentage of NK cells in each node of each sample as in panel G. X-axis and Y-axis show principal component 1 and principal component 2 that explain 31.9% and 24.1% of the total variance, respectively. Prediction ellipses indicate 95% probability that a new observation from the same group will fall inside the ellipse. N = 22 data points. (I) ViSNE clustering analysis of NK cells according to sample origin, showing expression levels of surface markers that were visibly different between 2009-0266 and 2012-0708.
Assessment of phenotype and function of mbIL21-expanded NK-cell infusion product (green) or PB NK cells patients. Mononuclear cells were isolated from PBMCs of patients on protocol 2009-0266 (without NK cells [red]) or 2012-0708 (with NK cells [green]) ∼28 days after stem cell transplant. Samples from patients on protocol 2012-0708 were obtained prior to receiving the third dose of NK cells (∼3 weeks after receiving the second dose). (A) Cytotoxicity against 721.221 targets. Cells were applied to the cytotoxicity assay at a 10:1 NK-to-target ratio (according to NK-cell content determined by immune phenotyping). (B) NK-cell responses to stimulation with 721.221 targets at a 2:1 NK-to-target ratio for 3 hours. Degranulation (CD107a), cytokine production, and CD62L cleavage were determined by mass cytometry. (C) Cytotoxicity and IFNγ production from panels A and B stratified according to cell dose received (low, ≤106/kg per dose; high, ≥107/kg per dose). P values shown for unpaired Student t test. (D) Differences in phenotype of NK cells at day +28 posttransplant as determined by mass cytometry. Shown are surface markers on NK cells from supplemental Figure 1 that were significantly different between 2009-0266 (no NK-cell infusions) and 2012-0708 (with NK-cell infusions) using multiple unpaired Student t-test comparisons followed by the 2-stage false discovery approach. Corrected P values are indicated as *P < .01, **P < .001, ***P < .0001, or ****P < .00001. (E) Heatmap with unsupervised clustering analysis of the NK-cell phenotypes from supplemental Figure 1 according to relative expression of each receptor in each sample. Sample origin is indicated along the top row: day +28 PB-NK samples from protocol 2009-0266 (red) or 2012-0708 (blue), or NK-cell product (green). (F) SPADE trees of NK-cell subsets present in the NK-cell product or peripheral blood at day +28 posttransplant. Given the small number of samples in each group, KIR expression was excluded from clustering to avoid bias from individual KIR and HLA genotypes. (G) Heatmap with unsupervised clustering analysis of the NK-cell subsets from each sample according to those identified in panel F (using only nodes constituting at least 1% of any 1 sample). Sample origin color coding is as in panel E. (H) Principal components analysis based on the percentage of NK cells in each node of each sample as in panel G. X-axis and Y-axis show principal component 1 and principal component 2 that explain 31.9% and 24.1% of the total variance, respectively. Prediction ellipses indicate 95% probability that a new observation from the same group will fall inside the ellipse. N = 22 data points. (I) ViSNE clustering analysis of NK cells according to sample origin, showing expression levels of surface markers that were visibly different between 2009-0266 and 2012-0708.
Phenotypic analysis of all NK cells from the 2 studies showed no significant differences in either percentage or median metal intensity (MMI) for any marker that survived multiple comparisons correction (supplemental Figure 1A-B).32 We then restricted analysis to only the mature single KIR+ cells for each donor in order to remove KIR genotype and licensing biases between the groups (supplemental Figure 1C-D). Significant differences were then observed for the percentage of single KIR+ NK cells expressing CD16, CD244 (2B4), NKG2C, CD223 (LAG3), CD62L, CD272 (BTLA), and CD357 (GITR), the first 4 being higher and the latter 3 lower in patients who received NK-cell infusions (Figure 4D). MMI differences for CD223, CD244, CD272, CD357, and NKG2C were also observed but did not remain significant after multiple comparison correction. Unsupervised clustering analysis on the basis of receptor expression was able to fully differentiate the NK cells in the infusion product from those obtained from PB (Figure 4E), but PB NK cells from the 2 different treatment groups (with and without NK cells) did not cluster discretely.
To further distinguish between PB-NK cells of the 2 groups, SPADE and ViSNE algorithms were used to identify and quantify NK-cell phenotypic subsets.32 SPADE analysis identified relatively few dominant phenotypes in each group of NK-cell sources (Figure 4F). No dominant subsets were common to both the infusion product and the PB-NK cells from patients, whereas there was close overlap between dominant subsets in PB-NK cells obtained from the 2 treatment groups (Figure 4F). Despite statistical significance between individual NK-cell markers of patients in the 2 studies (Figure 4D), unsupervised clustering analysis of the subpopulation proportions identified by SPADE for each cohort did not distinguish NK cells of patients between the 2 trials (Figure 4G). Likewise, a principal components analysis of the SPADE nodal data showed significant overlap between the 2 PB-NK-cell groups, although these were again clearly distinct from mbIL21-NK-cell infusion products (Figure 4H). Using ViSNE to broadly assess phenotypic variation, PB-NK cells from patients on the different studies aligned closely, with the exception of a distinct cluster having high CD4 and CD11a and absent NKG2A expression in patients who received NK-cell infusions (Figure 4I). As with SPADE, however, delineation between PB-NK cells from patients and the expanded NK-cell product was clear.
Transplant outcomes and comparison with retrospective controls
Engraftment, NRM, relapse rate, and survival.
Transplant outcomes for patients treated are summarized (Table 1). Median follow-up duration for survivors was 14.7 months (range, 8-25.1 months). All patients achieved primary engraftment (100%), all but 1 with 100% donor cell chimerism at day +28 posttransplant. The patient having mixed chimerism developed Parainfluenza pneumonia during the neutropenic period and subsequently died (day +85). The median time to neutrophil engraftment was 19 days (range, 15-27 days) and platelet engraftment 22 days (range, 13-39 days). Seven patients (54%) developed grade 2 aGVHD, controlled with topical steroids, budesonide, and/or systemic steroids. Only 5 of these patients required systemic steroids for aGVHD, which resolved rapidly. No grade 3-4 aGVHD nor cGVHD were observed. Only 1 patient relapsed (7.7%) at day +120 posttransplant. This patient was treated at the lowest investigated dose (1 × 105 NK cells per kilogram per dose) and was alive at last follow-up (day +582). One-year OS and DFS of the study group were 92% and 85%, respectively (Table 1). There were no toxicities >3 grade attributable to NK-cell infusion.
Comparison with retrospective control group of patients.
The control group of patients consisted of all 45 patients (median age, 45 years) with AML/MDS and CML in morphologic CR (≤5% bone marrow blasts) treated on the previous clinical trial with the same conditioning regimen but without NK cells (MDACC protocol 2009-0266).34 Characteristics of these patients are summarized in supplemental Table 1. All patients in this group achieved primary engraftment (100%), with a median time to neutrophil and platelet engraftment of 18 and 40 days, respectively. Grade 2-4 aGVHD in this group occurred in 14 of 44 patients (31.8%), grade 3-4 aGVHD in 1 of 41 patients (2.3%), and cGVHD occurred in 7 of 41 evaluable patients (17%). Nine patients (20%) died of NRM, 10 patients (22%) relapsed, and 25 patients (55.5%) were alive at last follow-up. Comparative outcomes between the current study and the control group of patients are presented in Figure 3.
Incidence of viral reactivation.
For patients treated with NK-cell infusions, 4 patients (30.8%) had cytomegalovirus (CMV) reactivation (2 of which received treatment with systemic steroids), and only 1 patient (7.7%) had asymptomatic BKV viruria (grade 1). Incidence in the control group was significantly higher for CMV reactivation (70.4%; P = .011) and trended higher for BKV cystitis (31.8%; P = .15), with 6 patients having grade 1, 6 patients grade 2, and 2 patients grade 3/4 hemorrhagic cystitis, respectively (Figure 3, panels G and F). Median CMV reactivation time was 28 days for patients treated without NK cells and 39 days for patients treated with NK cells (Figure 3G).35
Reconstitution of T-cell subsets.
Immunologic T-cell reconstitution was evaluated around days +28, +90, and +180 for patients in both groups (Figure 5). No significant differences were found in total white blood cell (WBC) count, CD3+ T cells, CD25+ regulatory T cells, CD45RA+ naive T cells, CD45RO memory T cells, or CD19+ B cells (Figure 4). Though not statistically significant, patients treated with NK cells tended to have higher NK-cell numbers at day +28 (median, 121.3 vs 77.4; P = .47), day +90 (median, 248.1 vs 159.0; P = .08), and day +180 (median, 287.7 vs 185.2; P = .12). Although no significant differences in absolute CD3+CD4+ cell numbers were observed, CD3+CD8+ T-cell numbers were significantly lower at day +28 in patients treated with NK cells (median, 0.15 vs 20.5; P < .001) (Figure 5E). Later recovery of CD3+CD4+ and CD3+CD8+ T-cell subsets was similar for both treatment groups (day +90 [CD4+: 183.4 vs 148.0, P = .99; and CD8+: 378.1 vs 291.3, P = .93] and day +180 (CD4+: 310.9 vs 245.2, P = .29; and CD8+: 673.2 vs 525.0, P = .76]).
Immunologic reconstitution of lymphocyte subsets in the first 6 months posttransplant for patients treated with and without NK cells. Immune subsets were determined by clinical flow cytometry. Absolute cell counts were calculated based on subset percentages and total WBC count obtained at the same time. Red, 2009-0266, previous clinical trial without NK cells; blue, 2012-0708, current phase 1 clinical trial. (A) Total WBC count. (B) Total lymphocyte count (CD3+). (C) CD56+CD3− NK cells. (D) CD3+CD4+ T cells. (E) CD3+CD8+ T cells. (F) CD3+CD25+ T-regulatory cells. (G) CD3+CD45RA+ naïve T cells. (H) CD+CD45RO+ memory T cells. (I) CD19+ B cells. D30, day 30 posttransplant; D90, day 90 posttransplant; day 180, day 180 posttransplant; WBC, total WBC count.
Immunologic reconstitution of lymphocyte subsets in the first 6 months posttransplant for patients treated with and without NK cells. Immune subsets were determined by clinical flow cytometry. Absolute cell counts were calculated based on subset percentages and total WBC count obtained at the same time. Red, 2009-0266, previous clinical trial without NK cells; blue, 2012-0708, current phase 1 clinical trial. (A) Total WBC count. (B) Total lymphocyte count (CD3+). (C) CD56+CD3− NK cells. (D) CD3+CD4+ T cells. (E) CD3+CD8+ T cells. (F) CD3+CD25+ T-regulatory cells. (G) CD3+CD45RA+ naïve T cells. (H) CD+CD45RO+ memory T cells. (I) CD19+ B cells. D30, day 30 posttransplant; D90, day 90 posttransplant; day 180, day 180 posttransplant; WBC, total WBC count.
Discussion
We report results of a phase 1 clinical trial in which multiple high doses of ex vivo–expanded NK cells were delivered perihaploidentical transplantation, with the goal of enhancing graft-versus-leukemia (GVL) activity and reducing risk of relapse.36-38 Large NK-cell doses were successfully generated and safely infused in this study with a promising improvement in relapse rate, immune reconstitution, and viral control.
Previous studies showed safety of apheresis-derived NK cells, but efficacy remains unclear. We established a method for ex vivo expansion of NK cells by stimulation with K562 Clone9.mbIL21 feeder cells to produce mbIL21-NK cells, with dramatically increased cytotoxicity and cytokine production.25 In this study, we evaluated the effect of multiple high doses of haploidentical NK cells expanded with IL-21 and 4-1BBL (up to 3 × 108/kg) infused peritransplant in a relatively small number of patients. We found no infusional reactions and no increased risk of severe aGVHD or cGVHD, in contrast to matched allogeneic NK cells expanded with IL-15 and 4-1BBL,16 confirming the safety of this cellular therapy product. Relapse was remarkably low, with only 1 of 13 patients relapsing at last follow-up, despite having poor-risk cytogenetics, high-risk molecular mutations, or primary induction failure, which predict high relapse rate posttransplant. Although a phase 1 trial is designed to assess safety and feasibility, these early clinical outcomes are provocative, and support our hypothesis that infusion of mbIL21-NK cells expanded from the same haploidentical donor may enhance the GVL effect. Importantly, this was observed regardless of NK alloreactivity or better KIR genotypes, as these features are available only for a minority of patients.
Adoptive immunotherapy with expanded NK cells in the peritransplant period (day −2 and day +7) also improved NK-cell immune reconstitution, and correlated with increased NK-cell cytotoxicity and cytokine production. This is particularly meaningful because cyclophosphamide was administered after the first infusion, and improved NK-cell function was detected before the third infusion of NK cells, 2 weeks after cryopreserved mbIL21-NK cells were infused (day +7) and while patients were on full immunosuppression. Additional data will be necessary to probe the persistence of fresh infusions (logistically more difficult) and to show that early discontinuation of immunosuppression (which increases risk of GVHD) is not required for the observed therapeutic effect.39 The better NK-cell numbers and function at day +28, however, may be independent of phenotype, which is largely similar between those treated or not treated with NK cells. Although we were able to detect an improvement in NK-cell number and function early posttransplant, which presumably contributed to an enhanced GVL effect, it was not possible to clearly differentiate between the expanded NK cells that were infused and those that derived from the donor stem cells by phenotype, nor was it possible to determine persistence by HLA typing or short tandem repeat chimerism analysis, as was done in other studies.40 We were only able to differentiate phenotypes between the 2 groups if the analysis was restricted to mature KIR+ subsets. These mature NK cells may represent persistent infused NK cells, or may represent a change caused by the infused cells in maturation of NK cells arising de novo from the new marrow.
Viral reactivation remains a significant complication after haploidentical transplantation with high rates of CMV reactivation and BK virus cystitis.35 In this study, we also observed a marked decrease in viral reactivation in the group treated with mbIL21-NK cells. Remarkably, the incidence of CMV reactivation was halved compared with the control group and none developed BKV cystitis. Previous studies demonstrated that NKG2C+ NK cells correlate with both control of CMV reactivation and GVL effect.32,33 In fact, NKG2C+ NK cells were significantly increased at day +28 in these patients. Although viral control may be mediated by cross-talk with adaptive immunity, this mechanism is contradicted by the apparent transient absence of CD8+ T-cell numbers on day +28. However, neither CD4+ T cells nor NK cells were lower at this time point.
In conclusion, this phase 1 clinical trial demonstrates that infusions of high doses of ex vivo–expanded donor-derived haploidentical NK cells in haploidentical bone marrow transplantation are safe and may be potentially effective in controlling leukemia relapse with no major toxicity. These results justify further clinical evaluation with phase 2 and 3 studies to assess the benefit of mbIL21 ex vivo–expanded NK cells in conjunction with haploidentical transplantation. Future studies will need to explore the scalability of NK-cell production required for larger multicenter studies and control production costs, which, so far, represent only a small fraction of transplant costs and should be competitive with new drug therapies that are emerging as posttransplant maintenance strategies.
Presented as an oral abstract at the 57th and 58th annual meetings of the American Society of Hematology, Orlando, FL, 5-8 December 2015 (abstract 102) and San Diego, CA, 3-6 December 2016 (abstract 500).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Robert Igarashi for critical review of the manuscript.
This work was supported in part by MD Anderson Cancer Center support grants from the National Institutes of Health, National Cancer Institute (P30 CA016672 and P01 CA49639), Leukemia & Lymphoma Society Translational Research Program Award 6149-14, the Cancer Prevention Research Institute of Texas (RP110553), The University of Texas MD Anderson Cancer Center AML Moonshot Program and High Impact Clinical Research Support Program, the McKee Family Foundation, and the Taylor Trudeau Cycle for Life Charitable Foundation.
Authorship
Contribution: S.O.C. contributed to study design, enrollment of patients on study, and interpretation of results, and wrote the manuscript; J.R.S. and C.J.D. performed the laboratory experiments and contributed to interpretation of laboratory data; R.B. contributed to trial design and statistical analysis; K.C. and D.W. contributed to HLA typing and donor selection; G.R. and J.C. contributed to data collection; D.S., A.G., and S.A. contributed to patient enrollment; I.K., K.R., and E.J.S. contributed to NK-cell manufacturing; D.A.L. contributed to study design, NK-cell manufacturing procedure, laboratory data and interpretation of results, and manuscript writing; R.E.C. contributed to study design, patient enrollment, interpretation of results, and manuscript writing; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: S.O.C. served as a consultant for MolMed and Spectrum Pharmaceuticals and has equity/leadership in CytoSen Therapeutics. D.A.L served as a consultant for Ziopharm Oncology, Courier Therapeutics, and Intellia Therapeutics, and has equity/leadership in CytoSen Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Stefan O. Ciurea, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: sciurea@mdanderson.org.
References
Author notes
D.A.L. and R.E.C. contributed equally to this study.

![Figure 3. Comparison of clinical outcomes between patients treated on protocols 2012-0708 (with NK cells [blue line]) and 2009-0266 (no NK cells [red line]). Data from 2009-0266 were censored to include patients with only AML/MDS and CML in morphologic CR at the time of transplant. (A) Time to neutrophil engraftment. (B) Time to platelet engraftment. (C) Cumulative incidence of grade 2-4 acute GVHD. (D) Cumulative incidence of grade 3-4 aGVHD. (E) Cumulative incidence of chronic GVHD limited + extensive. (F) Incidence of BKV cystitis. (G) Incidence of CMV reactivation. (H) Cumulative incidence of relapse. (I) Probability of progression-free survival. Kaplan-Meier survival curves were compared using the log-rank method. BKV, BK polyomavirus.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/16/10.1182_blood-2017-05-785659/6/m_blood785659f3.jpeg?Expires=1769117400&Signature=06m-COr5O26kKfodc5Ddpg9gvzezJ-bL0iXdP6WxiDqVDwgasEIbssL-GMhEYHng0wyfrUlttv5hIf3ektudKOt63T5NTbHR1ERHW9n10V9kkrY0fxeNNfEcT~pCoTqJv8--YgIaz~p~WhDXjnnP9TbQ76U00bsS9CBkTnkJg1OTih3uGMjzjWknmOUn8nqKn68rX1IXDHLVwnPNx-Q0qTgV0ca76TDJX0OfU~wWqRNsUzM5HGQjQSOysxtist6NGm0h2CuXKDbpEAOKVL4pR93fNnF7G6yqckjofEQO3vV95Cpnk0i81dAa~9DztKHLjE2XT1XSNum9LkFxcT9aeg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Assessment of phenotype and function of mbIL21-expanded NK-cell infusion product (green) or PB NK cells patients. Mononuclear cells were isolated from PBMCs of patients on protocol 2009-0266 (without NK cells [red]) or 2012-0708 (with NK cells [green]) ∼28 days after stem cell transplant. Samples from patients on protocol 2012-0708 were obtained prior to receiving the third dose of NK cells (∼3 weeks after receiving the second dose). (A) Cytotoxicity against 721.221 targets. Cells were applied to the cytotoxicity assay at a 10:1 NK-to-target ratio (according to NK-cell content determined by immune phenotyping). (B) NK-cell responses to stimulation with 721.221 targets at a 2:1 NK-to-target ratio for 3 hours. Degranulation (CD107a), cytokine production, and CD62L cleavage were determined by mass cytometry. (C) Cytotoxicity and IFNγ production from panels A and B stratified according to cell dose received (low, ≤106/kg per dose; high, ≥107/kg per dose). P values shown for unpaired Student t test. (D) Differences in phenotype of NK cells at day +28 posttransplant as determined by mass cytometry. Shown are surface markers on NK cells from supplemental Figure 1 that were significantly different between 2009-0266 (no NK-cell infusions) and 2012-0708 (with NK-cell infusions) using multiple unpaired Student t-test comparisons followed by the 2-stage false discovery approach. Corrected P values are indicated as *P < .01, **P < .001, ***P < .0001, or ****P < .00001. (E) Heatmap with unsupervised clustering analysis of the NK-cell phenotypes from supplemental Figure 1 according to relative expression of each receptor in each sample. Sample origin is indicated along the top row: day +28 PB-NK samples from protocol 2009-0266 (red) or 2012-0708 (blue), or NK-cell product (green). (F) SPADE trees of NK-cell subsets present in the NK-cell product or peripheral blood at day +28 posttransplant. Given the small number of samples in each group, KIR expression was excluded from clustering to avoid bias from individual KIR and HLA genotypes. (G) Heatmap with unsupervised clustering analysis of the NK-cell subsets from each sample according to those identified in panel F (using only nodes constituting at least 1% of any 1 sample). Sample origin color coding is as in panel E. (H) Principal components analysis based on the percentage of NK cells in each node of each sample as in panel G. X-axis and Y-axis show principal component 1 and principal component 2 that explain 31.9% and 24.1% of the total variance, respectively. Prediction ellipses indicate 95% probability that a new observation from the same group will fall inside the ellipse. N = 22 data points. (I) ViSNE clustering analysis of NK cells according to sample origin, showing expression levels of surface markers that were visibly different between 2009-0266 and 2012-0708.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/16/10.1182_blood-2017-05-785659/6/m_blood785659f4-1.jpeg?Expires=1769117400&Signature=Ydi~Lk04T9MZTlT02BKB2LcHhbAQnN1ObHnUx55Kbx4e7S26PwS9i~Os07q~AKMlsC1bikoYCq-rnd36NxyGdHzsgWtZoCvsLDk1Uxj1icSHxLmFvWn-ZyZYUsNUMcaQTpru97Pd5Yd3aVYl0um393AiTqHIrOLh5vjw7p0QmLquKJQAquABGC37cO5VBbAULkMK8vTgEEiIc2KXkjjyKkmnQHMVDd3D6~8Z0ei2BMQMUghBlA2LG-mb4WkibjmydhgXAkxiU-xldyGGrAOdmZqFL0Xq9cQHZhJuS6tJIyEyN3pj0ryX~6a8Wyx3MYuqZCKoCNohUqEgn6AO7aLMdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Assessment of phenotype and function of mbIL21-expanded NK-cell infusion product (green) or PB NK cells patients. Mononuclear cells were isolated from PBMCs of patients on protocol 2009-0266 (without NK cells [red]) or 2012-0708 (with NK cells [green]) ∼28 days after stem cell transplant. Samples from patients on protocol 2012-0708 were obtained prior to receiving the third dose of NK cells (∼3 weeks after receiving the second dose). (A) Cytotoxicity against 721.221 targets. Cells were applied to the cytotoxicity assay at a 10:1 NK-to-target ratio (according to NK-cell content determined by immune phenotyping). (B) NK-cell responses to stimulation with 721.221 targets at a 2:1 NK-to-target ratio for 3 hours. Degranulation (CD107a), cytokine production, and CD62L cleavage were determined by mass cytometry. (C) Cytotoxicity and IFNγ production from panels A and B stratified according to cell dose received (low, ≤106/kg per dose; high, ≥107/kg per dose). P values shown for unpaired Student t test. (D) Differences in phenotype of NK cells at day +28 posttransplant as determined by mass cytometry. Shown are surface markers on NK cells from supplemental Figure 1 that were significantly different between 2009-0266 (no NK-cell infusions) and 2012-0708 (with NK-cell infusions) using multiple unpaired Student t-test comparisons followed by the 2-stage false discovery approach. Corrected P values are indicated as *P < .01, **P < .001, ***P < .0001, or ****P < .00001. (E) Heatmap with unsupervised clustering analysis of the NK-cell phenotypes from supplemental Figure 1 according to relative expression of each receptor in each sample. Sample origin is indicated along the top row: day +28 PB-NK samples from protocol 2009-0266 (red) or 2012-0708 (blue), or NK-cell product (green). (F) SPADE trees of NK-cell subsets present in the NK-cell product or peripheral blood at day +28 posttransplant. Given the small number of samples in each group, KIR expression was excluded from clustering to avoid bias from individual KIR and HLA genotypes. (G) Heatmap with unsupervised clustering analysis of the NK-cell subsets from each sample according to those identified in panel F (using only nodes constituting at least 1% of any 1 sample). Sample origin color coding is as in panel E. (H) Principal components analysis based on the percentage of NK cells in each node of each sample as in panel G. X-axis and Y-axis show principal component 1 and principal component 2 that explain 31.9% and 24.1% of the total variance, respectively. Prediction ellipses indicate 95% probability that a new observation from the same group will fall inside the ellipse. N = 22 data points. (I) ViSNE clustering analysis of NK cells according to sample origin, showing expression levels of surface markers that were visibly different between 2009-0266 and 2012-0708.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/16/10.1182_blood-2017-05-785659/6/m_blood785659f4-2.jpeg?Expires=1769117400&Signature=XrsXEEjAJ6SU8B6eBQpW9n1YTAQia5p~OhsLsZGTf2wqpxRB5~Tf~tr4LHLaSqTQpGhWT-fDraGViGkGtYcdLTs9yKyFTTNW-E-vB~iVIZiaLFJSwlIANsTQ5LiSNUz2EvUhskx~nMx~WzfPIpNYxFPZ5Gnl6MxUHOy6nu4K~DG1SbOLnx-lfX4GkNv~nGwFjyguqtn34wyPXOLXAalVUl20PX4OYPazJ8f-iA6S7xcqi6Jemq6MBrTRCv9b145Na19ykgYFDuwn3kvm5cm~c5H1WljGPdjs0cg~1Hq0AOLT6SZxp~8mXkf8RUUfNKAF5Mg20rGOxY4k1hTZj3-JwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal