In this issue of Blood, Krämer et al have provided a long-term update on the outcomes of patients enrolled in the German CLL Study Group CLL3X trial who underwent a matched related or unrelated allogeneic hematopoietic cell transplantation (allo-HCT) with a reduced-intensity fludarabine/alkylator-based approach for high-risk chronic lymphocytic leukemia (HR-CLL).1
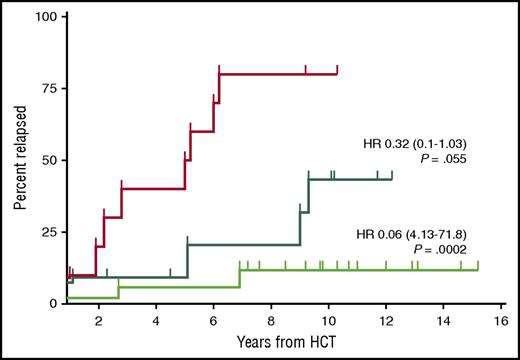
Relapse incidence of patients with MRD and event-free survival at 12 months after allo-HCT (n = 38). The red curve shows the relapse incidence for patients who were MRD positive at the 12-month landmark analysis after allo-HCT (n = 10). The dark green curve shows the relapse incidence of patients who became MRD negative immediately after transplantation and remained so at the 12-month landmark analysis (n = 11). The bright green curve shows the patients who became MRD negative only after immunosuppression tapering and remained so at the 12-month landmark analysis (n = 17). HR, hazard ratio (reference red curve). See Figure 2 in the article by Krämer et al that begins on page 1477.
Relapse incidence of patients with MRD and event-free survival at 12 months after allo-HCT (n = 38). The red curve shows the relapse incidence for patients who were MRD positive at the 12-month landmark analysis after allo-HCT (n = 10). The dark green curve shows the relapse incidence of patients who became MRD negative immediately after transplantation and remained so at the 12-month landmark analysis (n = 11). The bright green curve shows the patients who became MRD negative only after immunosuppression tapering and remained so at the 12-month landmark analysis (n = 17). HR, hazard ratio (reference red curve). See Figure 2 in the article by Krämer et al that begins on page 1477.
Although it is inherently less aggressive than acute leukemia and is frequently associated with an indolent course, this common adult leukemia comprises a wide spectrum of disease activity. As demonstrated in the initial report of Dreger et al,2 the strongest negative predictors for both progression-free survival (PFS) and overall survival (OS) in the study by Krämer et al were the use of alemtuzumab in the conditioning regimen and in refractory disease at the time of transplantation. Their results indicate a 20% non–relapse mortality (NRM) rate (9% for those without refractory disease or alemtuzumab treatment) and a 34% disease-free survival rate for all patients at 10 years. A landmark analysis of 32 patients who were alive and progression free at 6 years revealed a low rate of late relapse (18%), very low NRM (3%), and very high PFS (79%) for this population. These data indicate that long-term remissions with a low incidence of late relapse and low NRM are possible for HR-CLL patients, including those with TP53 abnormalities who did not seem to have adverse overall outcomes compared with the remainder of the group.
Additional analyses examined the important effect of minimal residual disease (MRD) and immune modulation on the eventual outcomes of these patients. Not surprisingly, persistent MRD at 1 year after transplantation was associated with poor outcomes, but patients who had MRD that was eradicated after withdrawal of immunosuppression actually did significantly better than those who became MRD negative immediately after transplantation and remained so at 1 year (see figure). This indicates the potency of the graft-versus-leukemia and immunotherapeutic effect of the donor graft and its critical role in long-term disease control. Unfortunately, this also correlated with a 73% incidence of some degree of chronic graft-versus-host disease (cGVHD), but the authors state that of those who remained in remission and alive at 6 years, 50% were not receiving immunosuppression therapy within 1 year of transplantation, indicating an extended period during which they remained in remission and free of cGVHD.
Perhaps the most important questions raised by the Krämer et al study relate to the current use of agents such as the B-cell receptor (bcr)/kinase inhibitor ibrutinib.3 These agents have clearly demonstrated dramatic and sustained responses in both standard-risk and high-risk patients without the short-term cytopenias or secondary hematologic malignancies associated with conventional chemotherapy combinations such as fludarabine, cyclophosphamide, and rituximab.4 The initial results with ibrutinib are promising, with 3-year PFS estimates of more than 90%, but complete remission remains the exception rather than the rule. The study also raises a question about long-term duration of response and the possibility of cure with these agents, even if they are administered continuously.5 Over time, mutations in the bcr pathway lead to the development of ibrutinib resistance and clinical relapse which, in many cases, is associated with generalized drug resistance and rapid progression.6 In addition to transplantation strategies, the advent of chimeric antigen receptor (CAR) T-cell–based approaches that are associated with short-term toxicities but deep initial remissions will likely also have a role in the treatment of these patients.7 These findings suggest that both cellular therapy and novel B-cell pathway inhibitors will continue to have a place in future treatment planning.
It is clear that low-risk therapies such as the bcr pathway inhibitors will have a progressively larger role in the majority of CLL patients, especially those who are older and less able to tolerate the rigors of cytokine release syndrome associated with CAR T cells or cGVHD associated with allo-HCT.8 At the same time, more intensive therapies will have a place in the treatment of younger patients, especially those with more aggressive and relapsed disease. As a result, there continues to be an important role for the cellular- and immune-based approaches embodied in allo-HCT or, as the results of longer follow-up become available, CAR T-cell approaches to provide cures for a greater number of patients with this disease. Additional follow-up on the quality of life of patients such as those described by Krämer et al and an estimate of the relative cost/benefit of cellular-based approaches compared with an estimated $138 000 per year average retail price9 for ibrutinib treatment will also be critical in optimizing treatment algorithms for this disease in the years to come.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal