Key Points
Laboratory parameters associated with increased bleeding were platelet counts ≤5 × 109/L, hematocrits ≤25%, INR >1.2, and aPTT >30 seconds.
Platelet and RBC transfusions on days with bleeding are often not sufficient to change bleeding outcomes on the following day.
Abstract
Bleeding remains a significant problem for many thrombocytopenic hematology/oncology patients in spite of platelet transfusions. Factors that might contribute to bleeding were analyzed for 16 320 patient-days on or after their first platelet transfusion in 1077 adult patients enrolled in the Platelet Dose (PLADO) trial. All patients had a greatly increased risk of bleeding at platelet counts of ≤5 × 109/L (odds ratio [OR], 3.1; 95% confidence interval [CI], 2.0-4.8) compared with platelet counts ≥81 × 109/L. Platelet counts between 6 × 109/L and 80 × 109/L were also associated with a somewhat elevated bleeding risk in patients receiving allogeneic stem cell transplants (SCTs) or chemotherapy but not in those undergoing autologous SCTs. Other significant laboratory predictors of bleeding were hematocrit ≤25% (OR, 1.29; 95% CI, 1.11-1.49), activated partial thromboplastin time (aPTT) 30 to ≤50 seconds (OR, 1.40; 95% CI, 1.08-1.81; P = .01), aPTT >50 seconds (OR, 2.34; 95% CI, 1.54-3.56), international normalized ratio (INR) 1.2 to 1.5 (OR, 1.46; 95% CI, 1.17-1.83), and INR >1.5 (OR, 2.05; 95% CI, 1.43-2.95). Transfusion of either platelets or red blood cells (RBCs) on days with bleeding was often not sufficient to change bleeding outcomes on the following day. Because bleeding occurred over a wide range of platelet counts among patients undergoing allogeneic SCT or chemotherapy and because platelet transfusions may not prevent bleeding, other risk factors may be involved. These may include low hematocrit and coagulation abnormalities. This trial was registered at www.clinicaltrials.gov as #NCT00128713.
Introduction
Prophylactic platelet transfusion is a mainstay of clinical care for patients with secondary hypoproliferative thrombocytopenia. However, the effectiveness of such therapy is variable, and as shown in prior studies, platelet transfusions may not always eliminate bleeding.1-3 Except at extremely low platelet counts, the degree of thrombocytopenia is not clearly associated with bleeding.2-6 Other factors may contribute to an increased risk of bleeding in thrombocytopenic patients, including the cause of thrombocytopenia, medications, underlying infection, and sepsis.7-9 Bleeding risk may also be related to coagulation abnormalities10 and low hematocrits.11-13
The Platelet Dose (PLADO) trial enrolled more than 1200 patients with hypoproliferative thrombocytopenia resulting from stem cell transplant (SCT) or chemotherapy for malignancy. The data set from the PLADO trial afforded a unique opportunity to examine the associations between platelet count, hematocrit, and coagulation factors (as assessed by international normalized ratio [INR], activated partial thromboplastin time [aPTT], and fibrinogen concentration) and the occurrence and grade of bleeding. In actively bleeding patients, the effects of platelet or red blood cell (RBC) transfusion on bleeding outcomes on the following day could also be evaluated.
Methods
As previously described,2 the PLADO study, conducted by the National Heart, Lung, and Blood Institute Transfusion Medicine/Hemostasis Clinical Research Network, was a multicenter randomized controlled trial of hospitalized patients expected to experience a period of hypoproliferative thrombocytopenia secondary to chemotherapy or SCT. Only patients age 18 years or older were included in this secondary analysis. Randomization was stratified by cause of thrombocytopenia: autologous or syngeneic SCT (AUTO stratum), allogeneic SCT (ALLO stratum), or chemotherapy for hematologic malignancy without SCT (CHEMO stratum). Patients were randomly assigned to 1 of 3 prophylactic doses: 1.1 × 1011 (low-dose [LD]), 2.2 × 1011 (medium-dose [MD]), or 4.4 × 1011 (high-dose [HD]) platelets per square meter of body surface area for each platelet transfusion. Additional eligibility criteria included INR and aPTT ≤1.3 × the upper limit of normal for the laboratory, fibrinogen ≥100 mg/dL, no prior platelet transfusions for thrombocytopenia during the current hospitalization, and no World Health Organization (WHO) bleeding grade ≥2 at eligibility assessment.
Transfusions
Platelet transfusions were given prophylactically for morning platelet counts of ≤10 × 109/L. The patient’s physician could change the transfusion trigger or dose based on clinical indications, with return to study guidelines as soon as possible. Local practice determined indications for RBC transfusion.
Clinical assessments
Supplemental Table 1 (available on the Blood Web site) gives the WHO definitions of grades 1 to 4 bleeding. Grade 1 is minor bleeding; grade 2 is more than minor bleeding but does not require RBC transfusion; grade 3 includes gross body cavity bleeding with organ dysfunction, visible blood with lumbar puncture but no central nervous system (CNS) symptoms, moderate hemodynamic instability, or RBC transfusion given to treat active bleeding; and grade 4 includes retinal bleeding with visual impairment, CNS symptoms with bloody lumbar puncture, CNS bleeding on imaging study, severe hemodynamic instability, and fatal bleeding. Research staff performed daily bleeding assessments by using physical examination, patient interview, and chart review, and they collected data using WHO criteria except for urine dipstick and stool guaiac tests. A computer algorithm used these data to generate each patient’s daily bleeding grade. For the analyses reported here, grade 2A bleeding was defined to be WHO grade 2 bleeding that was not solely the result of purpura. For a few patient-days, 1 or more of the daily bleeding outcomes could not be determined.
Daily platelet counts, hematocrits, and hemoglobins were obtained. Fibrinogen, aPTT, and INR were required only at baseline to determine study eligibility, but if any of these were performed as part of the patient’s management, the results were collected.
Study completion
Patients completed the study at 30 days from their first platelet transfusion, 10 days without a platelet transfusion, hospital discharge, death, or study withdrawal, whichever occurred first.
Statistical considerations
For this report, analyses were limited to the 1077 adult PLADO patients (age 18 years or older) who received at least 1 platelet transfusion. Analyses were also restricted to patient-days on or after the patient’s first platelet transfusion.
Baseline characteristics were summarized and tested for differences across the 3 dose groups and across the 3 strata with Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables. The distributions were summarized by using frequency (percentage) for categorical variables and mean (standard deviation) for continuous variables. Associations between bleeding grades (grade ≥2A, grade ≥3) and dose group, stratum, morning platelet count, hematocrit, fibrinogen, aPTT, or INR were examined by using logistic regression taking into account within-patient correlations. All statistical analyses were performed in SAS v9.4 and figures were plotted in R v3.1.1.
Protection for patients
Institutional review boards at each clinical site and the Data Coordinating Center approved the study, and each patient signed informed consent. A data and safety monitoring board reviewed data twice a year.
Results
Study population
Between 2004 and 2007, 26 sites enrolled 1351 patients of whom 1077 were age 18 years or older and received at least 1 platelet transfusion. These 1077 patients included 352 in the LD group, 355 in the MD group, and 370 in the HD group. There were 378 patients in the AUTO stratum, 413 in the ALLO stratum, and 286 in the CHEMO stratum. Baseline characteristics were generally well balanced between dose groups,2 but the strata differed on several baseline characteristics (Table 1). The large sample size resulted in statistically significant differences among baseline characteristics but none were considered clinically relevant.
Baseline characteristics by stratum
| Characteristic . | Stratum . | P* . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTO (n = 378) . | ALLO (n = 413) . | CHEMO (n = 286) . | |||||||||||
| No. . | % . | Mean . | SD . | No. . | % . | Mean . | SD . | No. . | % . | Mean . | SD . | ||
| Male sex | 238 | 63 | 252 | 61 | 155 | 54 | .06 | ||||||
| Age, y | 53 | 13 | 46 | 12 | 54 | 15 | <.001 | ||||||
| Body surface area, m2 | 1.95 | 0.23 | 1.95 | 0.23 | 1.91 | 0.23 | .02 | ||||||
| Platelet count, ×109/L | 47 | 36 | 50 | 38 | 37 | 25 | <.001 | ||||||
| Hematocrit, % | 29 | 3 | 29 | 4 | 28 | 4 | .002 | ||||||
| INR | 1.05 | 0.11 | 1.07 | 0.11 | 1.10 | 0.13 | <.001 | ||||||
| aPTT, s | 30 | 7 | 29 | 5 | 30 | 11 | .34 | ||||||
| Fibrinogen, mg/dL | 394 | 155 | 389 | 155 | 425 | 214 | .31 | ||||||
| Characteristic . | Stratum . | P* . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTO (n = 378) . | ALLO (n = 413) . | CHEMO (n = 286) . | |||||||||||
| No. . | % . | Mean . | SD . | No. . | % . | Mean . | SD . | No. . | % . | Mean . | SD . | ||
| Male sex | 238 | 63 | 252 | 61 | 155 | 54 | .06 | ||||||
| Age, y | 53 | 13 | 46 | 12 | 54 | 15 | <.001 | ||||||
| Body surface area, m2 | 1.95 | 0.23 | 1.95 | 0.23 | 1.91 | 0.23 | .02 | ||||||
| Platelet count, ×109/L | 47 | 36 | 50 | 38 | 37 | 25 | <.001 | ||||||
| Hematocrit, % | 29 | 3 | 29 | 4 | 28 | 4 | .002 | ||||||
| INR | 1.05 | 0.11 | 1.07 | 0.11 | 1.10 | 0.13 | <.001 | ||||||
| aPTT, s | 30 | 7 | 29 | 5 | 30 | 11 | .34 | ||||||
| Fibrinogen, mg/dL | 394 | 155 | 389 | 155 | 425 | 214 | .31 | ||||||
P values were calculated by using Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables.
Daily bleeding grades
Bleeding data were collected on 16 320 patient-days on or after the patients’ first platelet transfusion, with median days of 14 (LD), 13 (MD), and 14 (HD) (P > .05 for all pairwise comparisons). The median assessed days differed by stratum; 8 for AUTO, 16 for ALLO, and 19 for CHEMO (P < .001 for all two-way comparisons).
There were no differences between dose groups for any bleeding outcomes. Table 2 shows daily bleeding outcomes by patient-day for each stratum. There were significant differences between some strata for grade ≥2A bleeding. Patients in the ALLO stratum had bleeding on 21% of patient-days compared with 10% in the AUTO stratum and 11% in the CHEMO stratum (both P < .001); but the AUTO and CHEMO strata did not differ (P = .13). For grade ≥3 and grade 4 bleeding, there were no significant differences between strata.
Daily bleeding outcomes by stratum
| . | Stratum . | . | |||||
|---|---|---|---|---|---|---|---|
| AUTO (n = 3442) . | ALLO (n = 7143) . | CHEMO (n = 5735) . | P* (2 df) . | ||||
| No. . | % . | No. . | % . | No. . | % . | ||
| Grade ≥2A | <.001 | ||||||
| Yes | 341 | 10 | 1454 | 21 | 643 | 11 | |
| No | 3070 | 90 | 5610 | 79 | 5024 | 89 | |
| Missing data | 31 | 31 | 68 | ||||
| Grade ≥3 | .88 | ||||||
| Yes | 30 | 1 | 72 | 1 | 57 | 1 | |
| No | 3406 | 99 | 7065 | 99 | 5665 | 99 | |
| Missing data | 6 | 6 | 13 | ||||
| Grade 4 | .34 | ||||||
| Yes | 4 | <1 | 18 | <1 | 7 | <1 | |
| No | 3435 | >99 | 7117 | >99 | 5717 | >99 | |
| Missing data | 3 | 3 | 11 | ||||
| . | Stratum . | . | |||||
|---|---|---|---|---|---|---|---|
| AUTO (n = 3442) . | ALLO (n = 7143) . | CHEMO (n = 5735) . | P* (2 df) . | ||||
| No. . | % . | No. . | % . | No. . | % . | ||
| Grade ≥2A | <.001 | ||||||
| Yes | 341 | 10 | 1454 | 21 | 643 | 11 | |
| No | 3070 | 90 | 5610 | 79 | 5024 | 89 | |
| Missing data | 31 | 31 | 68 | ||||
| Grade ≥3 | .88 | ||||||
| Yes | 30 | 1 | 72 | 1 | 57 | 1 | |
| No | 3406 | 99 | 7065 | 99 | 5665 | 99 | |
| Missing data | 6 | 6 | 13 | ||||
| Grade 4 | .34 | ||||||
| Yes | 4 | <1 | 18 | <1 | 7 | <1 | |
| No | 3435 | >99 | 7117 | >99 | 5717 | >99 | |
| Missing data | 3 | 3 | 11 | ||||
The percentages expressed are based on the total days with non-missing data.
P values take into account within-person correlation.
Associations between morning platelet count and daily bleeding outcomes
The median morning platelet counts for patient-days with and without grade ≥2A bleeding were 21 × 109/L and 20 × 109/L, respectively, with identical 25th and 75th percentiles of 12 × 109/L and 37 × 109/L. Figure 1A shows that the percentage of patient-days with grade ≥2A bleeding ranged from 8% to 21% on the basis of the morning platelet count category. These percentages do not take into account within-person correlation (the tendency for patient-day outcomes to be more similar within a patient than between patients). Figure 1B shows the odds ratios (ORs) comparing each lower platelet count category to the reference category of ≥81 × 109/L. At platelet counts ≤80 × 109/L, most platelet count categories had significantly higher risk of grade ≥2A bleeding compared with the reference category, usually with P < .001. The risk of grade ≥2A bleeding was highest for the 1 × 109/L to 5 × 109/L category (OR, 3.1; 95% CI, 2.0-4.8). ORs ranged from 1.3 to 2.6 for categories between 6 × 109/L and 80 × 109/L, with no clear pattern of decreasing risk with increasing platelet counts. The associations between category of morning platelet count and grade ≥2A bleeding were similar for all 3 dose groups (interaction P = .84).
Relationship between morning platelet count and patient-days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A (grade 2A+) bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of ≥81 × 109/L, taking into account within-person correlation. The 16 df test for any association between morning platelet count category and grade ≥2A bleeding had P < .001. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 (grade 3+) bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of ≥81 × 109/L, taking into account within-person correlation. The 16 df test for any association between morning platelet count category and grade ≥3 bleeding had P = .85.
Relationship between morning platelet count and patient-days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A (grade 2A+) bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of ≥81 × 109/L, taking into account within-person correlation. The 16 df test for any association between morning platelet count category and grade ≥2A bleeding had P < .001. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 (grade 3+) bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of ≥81 × 109/L, taking into account within-person correlation. The 16 df test for any association between morning platelet count category and grade ≥3 bleeding had P = .85.
The median morning platelet count for patient-days without grade ≥3 bleeding was 20 × 109/L, with 25th and 75th percentiles of 12 × 109/L and 37 × 109/L. For patient-days with grade ≥3 bleeding, the median platelet count was 19 × 109/L, with 25th and 75th percentiles of 11 × 109/L and 38 × 109/L. There was no statistically significant relationship between the morning platelet count category and the occurrence of grade ≥3 bleeding (P = .85; Figure 1C-D).
Association between morning platelet count and daily bleeding within strata
There was a significant interaction (P = .03) between morning platelet count categories and stratum. Figure 2A shows the relationship between platelet count and grade ≥2A bleeding by stratum, not taking into account within-person correlations. Over a wide range of platelet counts, the ALLO stratum had a higher risk of bleeding than other strata. Figure 2B-D show ORs for each morning platelet count category vs the reference category of ≥81 × 109/L for the ALLO, AUTO, and CHEMO strata. In both the ALLO and the CHEMO strata, most categories had significantly higher risk than the reference category, but there was no clear pattern of decreasing bleeding with increasing platelet count in the range of 6 × 109/L to 80 × 109/L. For the AUTO stratum, only the 1 × 109/L to 5 × 109/L group had significantly higher bleeding risk than the reference category (P = .03).
Association between morning platelet count and grade ≥2A bleeding by stratum. (A) Unadjusted percentages of patient-days with grade ≥2A bleeding by stratum. (B-D) ORs (95% CIs), taking into account the within-patient correlation, comparing morning platelet count categories to the reference category of ≥81 × 109/L for (B) ALLO, (C) AUTO, and (D) CHEMO strata.
Association between morning platelet count and grade ≥2A bleeding by stratum. (A) Unadjusted percentages of patient-days with grade ≥2A bleeding by stratum. (B-D) ORs (95% CIs), taking into account the within-patient correlation, comparing morning platelet count categories to the reference category of ≥81 × 109/L for (B) ALLO, (C) AUTO, and (D) CHEMO strata.
Associations between morning hematocrit and daily bleeding outcomes
The median morning hematocrit for patient-days without grade ≥2A bleeding was 28% with 25th and 75th percentiles of 26% and 30%, and for patient-days with grade ≥2A bleeding, the median hematocrit was 27% with 25th and 75th percentiles of 25% and 29%. Figure 3A shows the relationship between morning hematocrit and whether grade ≥2A bleeding occurred that day. Figure 3B shows the ORs for each lower hematocrit category compared with the reference category of hematocrit >29%. Hematocrit was a significant predictor of grade ≥2A bleeding (overall 2 degrees of freedom [df] P = .002). Hematocrits of ≤25% had an OR of 1.29 (95% CI, 1.11-1.49) compared with the reference category. Hematocrits of 26% to 29% had an OR of 1.11 (95% CI, 0.98-1.26). There were no significant interactions between hematocrit category and stratum or between hematocrit category and dose group (interaction P = .82 and P = .18, respectively).
Relationship between morning hematocrit and percentage of patient-days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of hematocrit >29%, taking into account within-person correlation. The 2 df test for any association between morning hematocrit category and grade ≥2A bleeding had P = .002. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of hematocrit >29%, taking into account within-person correlation. The 2 df test for any association between morning hematocrit category and grade ≥3 bleeding had P < .001.
Relationship between morning hematocrit and percentage of patient-days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of hematocrit >29%, taking into account within-person correlation. The 2 df test for any association between morning hematocrit category and grade ≥2A bleeding had P = .002. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of hematocrit >29%, taking into account within-person correlation. The 2 df test for any association between morning hematocrit category and grade ≥3 bleeding had P < .001.
The median morning hematocrit value for patient-days without grade ≥3 bleeding was 28% with 25th and 75th percentiles of 26% and 30%, and for patient-days with grade ≥3 bleeding, the median hematocrit was 25% with 25th and 75th percentiles of 23% and 28%. Figure 3C shows the unadjusted relationship between morning hematocrit and whether grade ≥3 bleeding occurred. Figure 3D shows the OR for each category compared with the reference category of hematocrit >29%. Hematocrit was a significant predictor of grade ≥3 bleeding (overall 2 df P < .001). Similar to grade ≥2A bleeding, only days with hematocrit ≤25% were significantly different from the reference category with an OR of 4.88 (95% CI, 2.61-9.94). Hematocrits of 26% to 29% had an OR of 1.54 (95% CI, 0.81-2.96). The effect of hematocrit was similar across dose groups (interaction P = .13) and across strata (interaction P = .18).
Coagulation assays
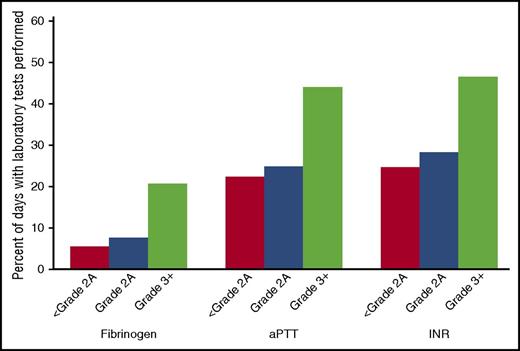
Fibrinogen, aPTT, and INR were more likely to be performed on days when patients were bleeding (Figure 4). Compared with days with grade <2A bleeding, each assay was more likely to be ordered on a day with grade 2A bleeding (P < .05), and even more likely to be ordered on a day with grade ≥3 bleeding (P < .003).
Relationship between bleeding grade and availability of fibrinogen, aPTT, and INR data. The y-axis indicates percentage of patient days with laboratory test performed among all patient days within specified bleeding grade.
Relationship between bleeding grade and availability of fibrinogen, aPTT, and INR data. The y-axis indicates percentage of patient days with laboratory test performed among all patient days within specified bleeding grade.
There were 949 patient-days with fibrinogen tests among 264 patients. Of these 949 patient-days, 46 had fibrinogen ≤200 mg/dL, 243 had fibrinogen between 200 and ≤400 mg/dL, 294 had fibrinogen between 400 and ≤600 mg/dL, and 366 had fibrinogen >600 mg/dL. There were no significant differences between the fibrinogen categories with respect to grade ≥2A bleeding (P = .86) or grade ≥3 bleeding (P = .81) (data not shown).
There were 3697 patient-days with aPTT tests among 676 patients. aPTT category was significantly related to the occurrence of grade ≥2A bleeding (P = .002). Figure 5A shows the percentage of days with grade ≥2A bleeding by aPTT category, and Figure 5B shows the OR for each category compared with the reference category of aPTT ≤30 seconds. Compared with the reference category, aPTT of 30 to ≤50 seconds was associated with a higher risk of bleeding (OR, 1.40; 95% CI, 1.08-1.81), and aPTT >50 seconds was associated with a still higher risk of bleeding (OR, 2.34; 95% CI, 1.54-3.56). aPTT category was not significantly associated with grade ≥3 bleeding (P = .40; Figure 5C-D), although there was some indication that aPTT >50 seconds may confer a higher risk (OR, 2.55; 95% CI, 0.90-7.21). However, there were very few patient-days with grade ≥3 bleeding in this category.
Relationship between aPTT category and percentage of days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of aPTT ≤30, taking into account within-person correlation. The 2 df test for any association between aPTT category and grade ≥2A bleeding had P = .002. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of aPTT ≤30, taking into account within-person correlation. The 2 df test for any association between aPTT category and grade ≥3 bleeding had P = .40.
Relationship between aPTT category and percentage of days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of aPTT ≤30, taking into account within-person correlation. The 2 df test for any association between aPTT category and grade ≥2A bleeding had P = .002. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of aPTT ≤30, taking into account within-person correlation. The 2 df test for any association between aPTT category and grade ≥3 bleeding had P = .40.
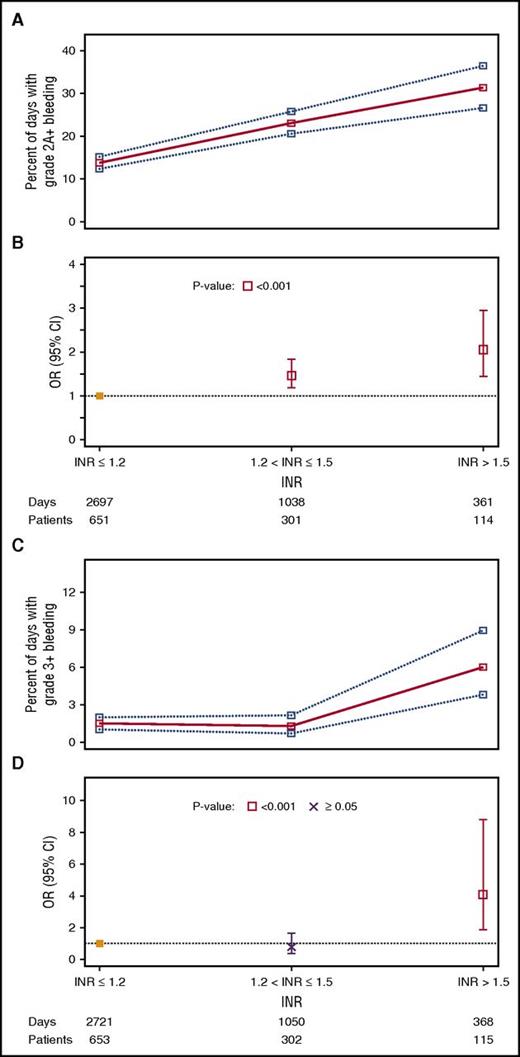
There were 4096 patient-days with INR results among 724 patients. INR category was significantly related to the occurrence of grade ≥2A bleeding (P < .001). Figure 6A shows the percentage of days with grade ≥2A bleeding by INR category, and Figure 6B shows the OR for each category compared with the reference category of INR ≤1.2. Compared with the reference category of INR ≤1.2, INR category 1.3 to 1.5 was associated with higher risk of grade ≥2A bleeding (OR, 1.46; 95% CI, 1.17-1.83), and INR >1.5 was associated with a still higher risk of bleeding (OR, 2.05; 95% CI, 1.43-2.95). INR category was also significantly associated with grade ≥3 bleeding (P = .04; Figure 6C-D), but the comparison with the reference group was significant only for the highest INR category. INRs between 1.3 and ≤1.5 had an OR of 0.79 (95% CI, 0.38-1.64). However, INR >1.5 had an OR of 4.08 (95% CI, 1.90-8.78).
Relationship between INR category and percentage of days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of INR ≤1.2, taking into account within-person correlation. The 2 df test for any association between INR category and grade ≥2A bleeding had P < .001. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of INR ≤1.2, taking into account within-person correlation. The 2 df test for any association between INR category and grade ≥3 bleeding had P = .04.
Relationship between INR category and percentage of days with bleeding outcomes. (A) Unadjusted percentages of patient-days (95% CIs) with grade ≥2A bleeding. (B) ORs (95% CIs) for grade ≥2A bleeding compared with the reference category of INR ≤1.2, taking into account within-person correlation. The 2 df test for any association between INR category and grade ≥2A bleeding had P < .001. (C) Unadjusted percentages of patient-days (95% CIs) with grade ≥3 bleeding. (D) ORs (95% CIs) for grade ≥3 bleeding compared with the reference category of INR ≤1.2, taking into account within-person correlation. The 2 df test for any association between INR category and grade ≥3 bleeding had P = .04.
Multipredictor models
Table 3 shows two multipredictor models for grade ≥2A bleeding. Model 1 includes stratum, platelet count category, and hematocrit (the 2 laboratory tests that were expected for every patient-day). Stratum, platelet count, and hematocrit remained significant predictors of bleeding, and the ORs for platelet count and hematocrit categories were similar to those in Figures 1B and 3B. Model 2 has aPTT and INR added. In this model, which is limited to patient-days with all 4 laboratory tests available, only stratum, aPTT, and INR are statistically significant.
Multipredictor models of laboratory predictors of grade ≥2A bleeding, taking into account within-person correlation
| Predictor . | Model 1* . | Model 2† . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | |
| Stratum | <.001 | <.001 | ||||
| ALLO | 1.00 | Ref. | 1.00 | Ref. | ||
| AUTO | 0.44 | 0.34-0.56 | <.001 | 0.38 | 0.26-0.55 | <.001 |
| CHEMO | 0.55 | 0.43-0.71 | <.001 | 0.55 | 0.39-0.77 | <.001 |
| Platelet count, ×109/L | <.001 | .55 | ||||
| 1-5 | 2.96 | 1.93-4.54 | <.001 | 1.79 | 0.86-3.75 | .12 |
| 6-10 | 2.02 | 1.41-2.87 | <.001 | 1.62 | 0.98-2.68 | .06 |
| 11-15 | 2.34 | 1.65-3.33 | <.001 | 1.61 | 0.95-2.72 | .08 |
| 16-20 | 2.21 | 1.56-3.13 | <.001 | 1.62 | 0.94-2.78 | .08 |
| 21-25 | 1.96 | 1.37-2.79 | <.001 | 1.52 | 0.91-2.54 | .11 |
| 26-30 | 1.89 | 1.33-2.68 | <.001 | 1.86 | 1.12-3.09 | .02 |
| 31-35 | 2.06 | 1.45-2.94 | <.001 | 1.68 | 0.96-2.94 | .07 |
| 36-40 | 1.82 | 1.26-2.63 | .001 | 1.50 | 0.86-2.61 | .16 |
| 41-45 | 1.91 | 1.33-2.74 | <.001 | 1.57 | 0.89-2.75 | .12 |
| 46-50 | 2.01 | 1.33-3.04 | <.001 | 1.81 | 0.97-3.37 | .06 |
| 51-55 | 2.10 | 1.38-3.19 | <.001 | 1.76 | 0.96-3.23 | .07 |
| 56-60 | 1.82 | 1.18-2.80 | .01 | 1.56 | 0.78-3.12 | .21 |
| 61-65 | 1.25 | 0.74-2.10 | .41 | 0.91 | 0.41-2.01 | .82 |
| 66-70 | 2.54 | 1.60-4.02 | <.001 | 2.28 | 0.97-5.34 | .06 |
| 71-75 | 1.64 | 0.91-2.98 | .10 | 0.96 | 0.41-2.25 | .93 |
| 76-80 | 1.67 | 0.91-3.04 | .10 | 1.46 | 0.60-3.55 | .40 |
| 81+ | 1.00 | Ref. | 1.00 | Ref. | ||
| Hematocrit, % | .02 | .35 | ||||
| ≤25 | 1.20 | 1.03-1.39 | .02 | 1.08 | 0.84-1.38 | .56 |
| 26-29 | 1.06 | 0.94-1.19 | .36 | 0.93 | 0.74-1.18 | .57 |
| >29 | 1.00 | Ref. | 1.00 | Ref. | ||
| aPTT | .04 | |||||
| ≤30 | 1.00 | Ref. | ||||
| 31-50 | 1.17 | 0.90-1.52 | .24 | |||
| >50 | 1.90 | 1.24-2.91 | .003 | |||
| INR | .01 | |||||
| ≤1.2 | 1.00 | Ref. | ||||
| 1.3-1.5 | 1.35 | 1.06-1.71 | .01 | |||
| >1.5 | 1.79 | 1.28-2.52 | <.001 | |||
| Predictor . | Model 1* . | Model 2† . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | |
| Stratum | <.001 | <.001 | ||||
| ALLO | 1.00 | Ref. | 1.00 | Ref. | ||
| AUTO | 0.44 | 0.34-0.56 | <.001 | 0.38 | 0.26-0.55 | <.001 |
| CHEMO | 0.55 | 0.43-0.71 | <.001 | 0.55 | 0.39-0.77 | <.001 |
| Platelet count, ×109/L | <.001 | .55 | ||||
| 1-5 | 2.96 | 1.93-4.54 | <.001 | 1.79 | 0.86-3.75 | .12 |
| 6-10 | 2.02 | 1.41-2.87 | <.001 | 1.62 | 0.98-2.68 | .06 |
| 11-15 | 2.34 | 1.65-3.33 | <.001 | 1.61 | 0.95-2.72 | .08 |
| 16-20 | 2.21 | 1.56-3.13 | <.001 | 1.62 | 0.94-2.78 | .08 |
| 21-25 | 1.96 | 1.37-2.79 | <.001 | 1.52 | 0.91-2.54 | .11 |
| 26-30 | 1.89 | 1.33-2.68 | <.001 | 1.86 | 1.12-3.09 | .02 |
| 31-35 | 2.06 | 1.45-2.94 | <.001 | 1.68 | 0.96-2.94 | .07 |
| 36-40 | 1.82 | 1.26-2.63 | .001 | 1.50 | 0.86-2.61 | .16 |
| 41-45 | 1.91 | 1.33-2.74 | <.001 | 1.57 | 0.89-2.75 | .12 |
| 46-50 | 2.01 | 1.33-3.04 | <.001 | 1.81 | 0.97-3.37 | .06 |
| 51-55 | 2.10 | 1.38-3.19 | <.001 | 1.76 | 0.96-3.23 | .07 |
| 56-60 | 1.82 | 1.18-2.80 | .01 | 1.56 | 0.78-3.12 | .21 |
| 61-65 | 1.25 | 0.74-2.10 | .41 | 0.91 | 0.41-2.01 | .82 |
| 66-70 | 2.54 | 1.60-4.02 | <.001 | 2.28 | 0.97-5.34 | .06 |
| 71-75 | 1.64 | 0.91-2.98 | .10 | 0.96 | 0.41-2.25 | .93 |
| 76-80 | 1.67 | 0.91-3.04 | .10 | 1.46 | 0.60-3.55 | .40 |
| 81+ | 1.00 | Ref. | 1.00 | Ref. | ||
| Hematocrit, % | .02 | .35 | ||||
| ≤25 | 1.20 | 1.03-1.39 | .02 | 1.08 | 0.84-1.38 | .56 |
| 26-29 | 1.06 | 0.94-1.19 | .36 | 0.93 | 0.74-1.18 | .57 |
| >29 | 1.00 | Ref. | 1.00 | Ref. | ||
| aPTT | .04 | |||||
| ≤30 | 1.00 | Ref. | ||||
| 31-50 | 1.17 | 0.90-1.52 | .24 | |||
| >50 | 1.90 | 1.24-2.91 | .003 | |||
| INR | .01 | |||||
| ≤1.2 | 1.00 | Ref. | ||||
| 1.3-1.5 | 1.35 | 1.06-1.71 | .01 | |||
| >1.5 | 1.79 | 1.28-2.52 | <.001 | |||
OR, odds ratio; Ref., reference.
Grade ≥2A bleeding days/total days = 2403/15 979 = 15%.
Grade ≥2A bleeding days/total days = 612/3,604 = 17%.
Table 4 shows a multipredictor model for grade ≥3 bleeding, including stratum, platelet count, and hematocrit. Similar to the one-predictor models, stratum and platelet count were not significantly associated with grade ≥3 bleeding, but hematocrit was, with the lowest category at greatly increased risk.
Multipredictor model of laboratory predictors of grade ≥3 bleeding, taking into account within-person correlation
| Predictor . | Model 1* . | ||
|---|---|---|---|
| OR . | 95% CI . | P . | |
| Stratum | .91 | ||
| ALLO | 1.00 | Ref. | |
| AUTO | 1.09 | 0.62-1.91 | .76 |
| CHEMO | 1.11 | 0.66-1.88 | .69 |
| Platelet count, ×109/L | .85 | ||
| 1-5 | 1.10 | 0.35-3.44 | .86 |
| 6-10 | 0.67 | 0.30-1.50 | .33 |
| 11-15 | 0.70 | 0.31-1.56 | .38 |
| 16-20 | 0.77 | 0.36-1.64 | .51 |
| 21-25 | 0.87 | 0.37-2.03 | .75 |
| 26-30 | 0.94 | 0.41-2.17 | .89 |
| 31-35 | 0.39 | 0.12-1.30 | .13 |
| 36-40 | 0.36 | 0.09-1.37 | .13 |
| 41-45 | 0.84 | 0.31-2.31 | .74 |
| 46-50 | 0.58 | 0.15-2.24 | .43 |
| 51-55 | 1.01 | 0.33-3.07 | .98 |
| 56-60 | 1.27 | 0.46-3.51 | .65 |
| 61-65 | 1.09 | 0.27-4.34 | .90 |
| 66-70 | 0.98 | 0.22-4.29 | .97 |
| 71-75 | 1.85 | 0.31-11.07 | .50 |
| 76-80 | 0.70 | 0.06-8.75 | .78 |
| 81+ | 1.00 | Ref. | |
| Hematocrit, % | <.001 | ||
| ≤25 | 5.09 | 2.65-9.79 | <.001 |
| 26-29 | 1.55 | 0.80-3.01 | .20 |
| >29 | 1.00 | Ref. | |
| Predictor . | Model 1* . | ||
|---|---|---|---|
| OR . | 95% CI . | P . | |
| Stratum | .91 | ||
| ALLO | 1.00 | Ref. | |
| AUTO | 1.09 | 0.62-1.91 | .76 |
| CHEMO | 1.11 | 0.66-1.88 | .69 |
| Platelet count, ×109/L | .85 | ||
| 1-5 | 1.10 | 0.35-3.44 | .86 |
| 6-10 | 0.67 | 0.30-1.50 | .33 |
| 11-15 | 0.70 | 0.31-1.56 | .38 |
| 16-20 | 0.77 | 0.36-1.64 | .51 |
| 21-25 | 0.87 | 0.37-2.03 | .75 |
| 26-30 | 0.94 | 0.41-2.17 | .89 |
| 31-35 | 0.39 | 0.12-1.30 | .13 |
| 36-40 | 0.36 | 0.09-1.37 | .13 |
| 41-45 | 0.84 | 0.31-2.31 | .74 |
| 46-50 | 0.58 | 0.15-2.24 | .43 |
| 51-55 | 1.01 | 0.33-3.07 | .98 |
| 56-60 | 1.27 | 0.46-3.51 | .65 |
| 61-65 | 1.09 | 0.27-4.34 | .90 |
| 66-70 | 0.98 | 0.22-4.29 | .97 |
| 71-75 | 1.85 | 0.31-11.07 | .50 |
| 76-80 | 0.70 | 0.06-8.75 | .78 |
| 81+ | 1.00 | Ref. | |
| Hematocrit, % | <.001 | ||
| ≤25 | 5.09 | 2.65-9.79 | <.001 |
| 26-29 | 1.55 | 0.80-3.01 | .20 |
| >29 | 1.00 | Ref. | |
Grade ≥3 bleeding days/total days = 155/16 129 = 1%.
Platelet transfusion
There were 2181 patients-days with exactly grade 2A bleeding that had bleeding data available on the following day. As shown in Table 5, on 1327 (61%) of these days, the patient received at least 1 platelet transfusion. Among these 1327 patient-days, 790 patients (60%) still had grade ≥2A bleeding the following day. Among the 854 patient-days without a platelet transfusion, only 445 patients (52%) still had grade ≥2A bleeding the following day. Taking into account within-person correlation, platelet transfusion was associated with significantly higher risk of continued grade ≥2A bleeding (OR, 1.28; 95% CI, 1.06-1.54). Adjusting for stratum or dose group did not change the results regarding the association of platelet transfusion with the next day’s bleeding grade. As shown in Table 5, platelet transfusions were more likely to be given on days with lower platelet counts. However, the relationship between platelet transfusion and next-day bleeding did not differ significantly between platelet count categories (interaction P = .34), and platelet transfusion was not associated with a significant improvement in next-day bleeding for any platelet count category.
Association between platelet transfusion on the day of grade 2A bleeding and grade ≥2A bleeding the following day, within morning platelet count subgroups, taking into account within-person correlation
| Morning platelet count . | Days with grade 2A bleeding . | Days with platelet transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days with platelet transfusion . | Days without platelet transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days without platelet transfusion . | OR* . | 95% CI . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |||||
| All platelet counts, ×109/L | 2181† | 1327 | 61 | 790 | 60 | 854 | 39 | 445 | 52 | 1.28 | 1.06-1.54 | .01 |
| 1-10 | 420 | 402 | 96 | 228 | 57 | 18 | 4 | 8 | 44 | 2.10 | 0.74-5.91 | .16 |
| 11-20 | 666 | 410 | 62 | 224 | 55 | 256 | 38 | 123 | 48 | 1.20 | 0.86-1.69 | .28 |
| 21-30 | 379 | 201 | 53 | 126 | 63 | 178 | 47 | 93 | 52 | 1.45 | 0.93-2.25 | .10 |
| 31-40 | 245 | 113 | 46 | 77 | 68 | 132 | 54 | 72 | 55 | 1.61 | 0.90-2.89 | .11 |
| 41-50 | 171 | 92 | 54 | 63 | 68 | 79 | 46 | 45 | 57 | 0.98 | 0.47-2.06 | .96 |
| 51-60 | 125 | 59 | 47 | 43 | 73 | 66 | 53 | 38 | 58 | 1.62 | 0.78-3.39 | .20 |
| 61-70 | 63 | 21 | 33 | 15 | 71 | 42 | 67 | 25 | 60 | 2.10 | 0.80-5.47 | .13 |
| 71-80 | 38 | 13 | 34 | 5 | 38 | 25 | 66 | 13 | 52 | 0.53 | 0.12-2.27 | .39 |
| 81+ | 67 | 12 | 18 | 6 | 50 | 55 | 82 | 26 | 47 | 1.33 | 0.42-4.16 | .63 |
| Morning platelet count . | Days with grade 2A bleeding . | Days with platelet transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days with platelet transfusion . | Days without platelet transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days without platelet transfusion . | OR* . | 95% CI . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |||||
| All platelet counts, ×109/L | 2181† | 1327 | 61 | 790 | 60 | 854 | 39 | 445 | 52 | 1.28 | 1.06-1.54 | .01 |
| 1-10 | 420 | 402 | 96 | 228 | 57 | 18 | 4 | 8 | 44 | 2.10 | 0.74-5.91 | .16 |
| 11-20 | 666 | 410 | 62 | 224 | 55 | 256 | 38 | 123 | 48 | 1.20 | 0.86-1.69 | .28 |
| 21-30 | 379 | 201 | 53 | 126 | 63 | 178 | 47 | 93 | 52 | 1.45 | 0.93-2.25 | .10 |
| 31-40 | 245 | 113 | 46 | 77 | 68 | 132 | 54 | 72 | 55 | 1.61 | 0.90-2.89 | .11 |
| 41-50 | 171 | 92 | 54 | 63 | 68 | 79 | 46 | 45 | 57 | 0.98 | 0.47-2.06 | .96 |
| 51-60 | 125 | 59 | 47 | 43 | 73 | 66 | 53 | 38 | 58 | 1.62 | 0.78-3.39 | .20 |
| 61-70 | 63 | 21 | 33 | 15 | 71 | 42 | 67 | 25 | 60 | 2.10 | 0.80-5.47 | .13 |
| 71-80 | 38 | 13 | 34 | 5 | 38 | 25 | 66 | 13 | 52 | 0.53 | 0.12-2.27 | .39 |
| 81+ | 67 | 12 | 18 | 6 | 50 | 55 | 82 | 26 | 47 | 1.33 | 0.42-4.16 | .63 |
The interaction between the 9 morning platelet count categories and platelet transfusion was not significant (P = .34).
Comparing platelet transfusion to no platelet transfusion.
Seven days had missing data for morning platelet count and are not included in the rows below.
There were 147 patient-days with grade ≥3 bleeding that had bleeding data available for the following day. For the 113 patient-days (77%) with at least 1 platelet transfusion, 22% had grade ≥3 bleeding the following day compared with 12% of the 34 patient-days without a platelet transfusion. This association between platelet transfusion and the following day’s bleeding grade was not statistically significant (P = .31).
RBC transfusion
Of the 2181 patient-days with exactly grade 2A bleeding and bleeding data available for the following day, 455 patient-days (21%) had at least 1 RBC transfusion on the day of grade 2A bleeding. As shown in the first row of Table 6, on 276 (61%) of these 455 patient-days, the patient still had grade ≥2A bleeding on the following day. Among the 1726 patient-days without an RBC transfusion on the day of grade 2A bleeding, only 56% of patients still had grade ≥2A bleeding the following day. However, the association between whether or not any RBC transfusion was given on the day of grade 2A bleeding and the following day, bleeding grade was not statistically significant in a model taking into account within-person correlation (OR, 1.20; 95% CI, 0.98-1.47). As shown in Table 6, RBC transfusions were more likely to be given on days with lower hematocrits, but there was no difference between hematocrit categories in the relationship between RBC transfusion and next-day bleeding (interaction P = .60), and RBC transfusion was not associated with reduced next-day bleeding in any hematocrit category.
Association between RBC transfusion on the day of grade 2A bleeding and grade ≥2A bleeding the following day, within hematocrit subgroups, taking into account within-person correlation
| Hematocrit category . | Days with hematocrit data on day of grade 2A bleeding . | Days with RBC transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days with RBC transfusion . | Days without RBC transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days without RBC transfusion . | OR* . | 95% CI . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | No. . | % . | No. . | % . | No. . | % . | No. . | |||||
| All hematocrit values | 2181† | 455 | 21 | 276 | 61 | 1726 | 79 | 959 | 56 | 1.20 | 0.98-1.47 | .08 |
| ≤25 | 524 | 296 | 56 | 182 | 61 | 228 | 44 | 144 | 63 | 1.02 | 0.74-1.41 | .91 |
| 25-29 | 1049 | 123 | 12 | 70 | 57 | 926 | 88 | 513 | 55 | 1.03 | 0.71-1.49 | .88 |
| >29 | 585 | 30 | 5 | 19 | 63 | 555 | 95 | 296 | 53 | 1.79 | 0.81-3.96 | .15 |
| Hematocrit category . | Days with hematocrit data on day of grade 2A bleeding . | Days with RBC transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days with RBC transfusion . | Days without RBC transfusion on the day of grade 2A bleeding . | Next day bleeding grade ≥2A after days without RBC transfusion . | OR* . | 95% CI . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | No. . | % . | No. . | % . | No. . | % . | No. . | |||||
| All hematocrit values | 2181† | 455 | 21 | 276 | 61 | 1726 | 79 | 959 | 56 | 1.20 | 0.98-1.47 | .08 |
| ≤25 | 524 | 296 | 56 | 182 | 61 | 228 | 44 | 144 | 63 | 1.02 | 0.74-1.41 | .91 |
| 25-29 | 1049 | 123 | 12 | 70 | 57 | 926 | 88 | 513 | 55 | 1.03 | 0.71-1.49 | .88 |
| >29 | 585 | 30 | 5 | 19 | 63 | 555 | 95 | 296 | 53 | 1.79 | 0.81-3.96 | .15 |
The interaction between the three hematocrit categories and RBC transfusion was not significant (P = .60).
Comparing RBC transfusion to no RBC transfusion.
Twenty-three days had missing hematocrit and are not included in the rows below.
Among the 147 patient-days with grade ≥3 bleeding that had bleeding data available for the following day, a red blood cell transfusion was given on 127 days (86%), and grade ≥3 bleeding continued on 21% of the following days compared with 2 (10%) of the 20 days with no RBC transfusion. The association between RBC transfusion given on the day of grade ≥3 bleeding and the following day bleeding grade was not statistically significant (OR, 2.14; 95% CI, 0.50-11.59).
Discussion
Hypoproliferative thrombocytopenia is a well-recognized complication of HD chemotherapy and hematopoietic SCT. Use of prophylactic platelet transfusions is widely accepted as standard of care to reduce the risk of clinically significant bleeding. However, recent examinations of this practice have raised several questions including whether or not a prophylactic platelet transfusion strategy to prevent bleeding is superior to therapeutic-only platelet transfusions to treat active bleeding.5,6 In addition, there are other factors (either laboratory or clinical) that may contribute to the risk of bleeding in thrombocytopenic patients. Our secondary analyses of PLADO Trial data using models with single laboratory predictors and models including multiple laboratory predictors suggest that platelet counts, hematocrits, coagulation factors, and clinical treatment categories may all predict increased risk of bleeding.
At morning platelet counts between 6 × 109/L and 80 × 109/L, there were fairly similar risks of grade ≥2A bleeding within each treatment stratum. Patients in the ALLO stratum had higher risks of grade ≥2A bleeding compared with patients in the AUTO and CHEMO strata, likely reflecting direct toxicity to the vascular endothelium and comorbid adverse effects of therapeutic regimens. At very low platelet counts of ≤5 × 109/L, patients in all 3 strata had a significantly higher bleeding risk compared with the reference category. These data are in direct contrast to data from articles by Webert et al9 and Estacourt et al14 who observed in smaller cohorts of patients with hematologic malignancies a reduction in bleeding risk for every 10 × 109/L increase in platelet count.
As has been reported by others, hematocrit was inversely associated with risk of bleeding.11,13 On patient days with hematocrit values ≤25%, there were increased risks for grade ≥2A and grade ≥3 bleeding compared with patient days with hematocrit values >29%. These observations are entirely consistent with animal models as well as human studies demonstrating correlation between prolonged bleeding times and hematocrit values.11-13,15 A caveat to these data are the fact that an RBC transfusion given for bleeding qualifies for grade ≥3 bleeding, thus it is important to be circumspect about causality.
Analysis of coagulation assays demonstrated an increased overall risk for bleeding for patients with abnormal INR and aPTT. However, these data should be interpreted with caution because the study did not require any coagulation assays after enrollment. This makes it difficult to accurately assess the association of these parameters with bleeding in the PLADO patients or the extent to which adjusting for them impacts the apparent association of other parameters with bleeding. Others have demonstrated no association between bleeding risk and functional assessment of coagulation by either standard coagulation assays or thromboelastography after adjustment for platelet count.14 Therefore, the observed association between increased bleeding risk and physician-ordered coagulation assays may simply relate to the clinical team’s assessment of a patient’s current bleeding that triggered the laboratory assessment.
There is increasing evidence supporting consideration of a risk-stratified approach to platelet transfusion management of hypoproliferative thrombocytopenia.5,6,16 Our secondary analysis demonstrated that patients in the AUTO stratum had lower risk for bleeding and thus represent a patient population for which a therapeutic-only platelet transfusion approach could be considered. However, even patients in the AUTO stratum had a high risk of bleeding at platelet counts ≤5 × 109/L. In addition, all patients in the PLADO trial received prophylactic platelet transfusions for morning counts ≤10 ×109/L, so we cannot determine what the effect of a therapeutic-only strategy would have been overall or in any of the 3 strata.
The articles by Wandt et al5 and Stanworth et al6 have findings remarkably similar to ours with respect to increased bleeding risk in patients treated with chemotherapy or allogeneic transplant vs patients undergoing autologous SCT. In both studies, the proportion of patients randomly assigned to therapeutic platelet transfusion experienced more days with bleeding compared with those receiving prophylactic treatment, but higher rates of grade 3 or 4 bleeding were observed in patients receiving chemotherapy or undergoing allogeneic SCT. These studies show that prophylactic platelet transfusions are effective, at least in certain patient populations, but clearly, treatment-related factors contribute to increased bleeding risk. Until these are better understood, the continuing use of a prophylactic platelet transfusion strategy for all patients with hypoproliferative thrombocytopenia seems prudent.
We sought to assess the impact of platelet and RBC transfusion on bleeding outcomes. Patients who received a platelet transfusion on a day with grade 2A bleeding were significantly more likely to have bleeding of at least grade 2A the next day, compared with patients who did not receive a platelet transfusion on a day with grade 2A bleeding. However, these results cannot be interpreted as proof that platelet transfusions do not reduce bleeding on the following day. Clinical teams may have elected to order platelet transfusions for patients judged to be at higher risk of continued or worsening bleeding the next day than for patients judged to be at lower risk. This introduces confounding by indication. Some patients who did receive a platelet transfusion on a day with grade 2A bleeding may have experienced better outcomes than they would have had if they had not received the platelet transfusion. The PLADO trial collected no data on the reasons for deciding whether to order a platelet transfusion on a particular patient-day, nor did it collect data on other daily risk factors for bleeding except for hematocrits. Therefore, we could not perform analyses to address these potential confounders. Similar observations apply to the relationship between RBC transfusion and improvement of bleeding grade. Furthermore, because the bleeding risk was constant over a broad range of platelet counts, it is not surprising that a minor improvement in platelet count associated with a platelet transfusion may not affect the next day’s bleeding grade. Similarly, minor improvement in hematocrit through RBC transfusion may not affect the next day’s bleeding grade. This is further supported by the observation that the median platelet counts and hematocrits on days with and without bleeding were similar.
The authors acknowledge several limitations of this study, including the small sample size for patients experiencing grades 3 and 4 bleeding. This precluded further analysis of the potential risk factors for higher bleeding grades, serving to remind us that further studies are required to elucidate underlying pathologies that may contribute to these rare occurrences.17 Another limitation relates to the fact that detailed information on chemotherapeutic treatment regimens and preparative transplant protocols were not collected, thus precluding the analysis of their possible contribution to the observed bleeding events. However, bleeding outcomes differed between treatment strata, suggesting that these factors resulted in significant effects on bleeding risks. Finally, the timing of bleeding events on a given day was not recorded, so it is not possible to tell whether a bleeding event occurred before or after a particular platelet or RBC transfusion or laboratory test.
In conclusion, the secondary analyses presented in this article attempt to further elucidate risk factors for bleeding in the setting of hypoproliferative thrombocytopenia. As described, increased overall risk for bleeding correlated with treatment stratum, profoundly low platelet counts (≤5 × 109/L), hematocrit of ≤25%, INR >1.2, and aPTT >30 seconds. The findings echo those reported by others in demonstrating that a large percentage of patients with hypoproliferative thrombocytopenia experience grade 2A bleeding16 and a minority experience grade 3 and/or 4 bleeding. Given the attendant risks of platelet transfusion (adverse reactions, including septic transfusion reactions), further exploration of the impact of treatment-related risk factors, and careful assessment of posttransfusion impact on bleeding is encouraged. In addition, we are in agreement with others regarding the need to develop alternatives to platelet transfusion therapy to improve hemostasis during the period of hypoproliferative thrombocytopenia experienced by patients undergoing therapy for hematologic malignancies.17
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Transfusion Medicine/Hemostasis Clinical Trials Network investigators, study coordinators, research staff, and patients who participated in this study.
This study was supported by National Heart, Lung, and Blood Institute, National Institutes of Health grants HL072268 (Data Coordinating Center at New England Research Institutes), HL072033 (Case Western Reserve University), HL072291 (Children’s Hospital Boston), HL072196 (Cornell University), HL072289 (Duke University), HL072248 (Emory University), HL072191 (Johns Hopkins University), HL072299 (Massachusetts General Hospital), HL072305 (Puget Sound Blood Center), HL072274 (Tulane University), HL072028 (University of Iowa), HL072359 (University of Maryland), HL072072 (University of Minnesota), HL072355 (University of North Carolina), HL072283 (University of Oklahoma), HL072346 (University of Pennsylvania), HL072331 (University of Pittsburgh), and HL072290 (Blood Center of Wisconsin).
Authorship
Contribution: L.U., S.F.A., T.G., and S.J.S. designed the research; L.U., T.G., and S.J.S. performed the research; L.U., S.F.A., T.G., T.H.H., R.W.H., and S.S. analyzed the data; and L.U., S.F.A., T.G., T.H.H., and S.J.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lynne Uhl, Division of Laboratory Medicine, Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215; e-mail: luhl@bidmc.harvard.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal