In this issue of Blood, Rasche et al provide the first evidence of the biological basis underlying the occurrence of false-negative scans with use of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in newly diagnosed transplant-eligible multiple myeloma (MM) patients.1
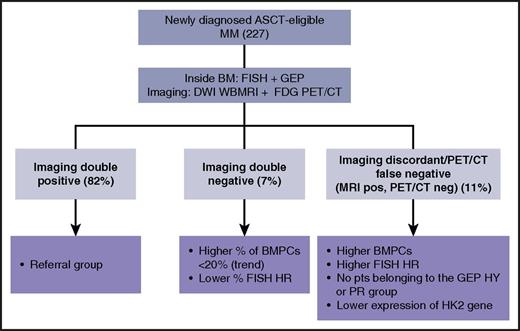
Assessment of newly diagnosed MM inside and outside the BM. ASCT, autologous stem cell transplantation; FISH, fluorescence in situ hybridization; HK2, hexokinase-2, HR, high risk; HY, hyperdiploid; neg, negative; pos, positive; PR, proliferative; pts, patients; WBMRI, whole-body magnetic resonance imaging.
Assessment of newly diagnosed MM inside and outside the BM. ASCT, autologous stem cell transplantation; FISH, fluorescence in situ hybridization; HK2, hexokinase-2, HR, high risk; HY, hyperdiploid; neg, negative; pos, positive; PR, proliferative; pts, patients; WBMRI, whole-body magnetic resonance imaging.
According to the updated diagnostic criteria for MM,2 the novel imaging techniques, including whole-body low-dose CT, magnetic resonance imaging (MRI), and PET/CT, are now considered a valuable tool for the diagnostic workup of MM because of their higher sensitivity and ability to detect bone damage at an earlier phase than whole-body radiograph. Several studies have demonstrated the usefulness of FDG-PET/CT at diagnosis, reporting a sensitivity and specificity for detection of bone lesions in the range between 80% and 100%. Moreover, functional imaging techniques, such as PET and MRI, are able to distinguish between metabolically active and inactive sites of clonal proliferating plasma cells (PCs), thus allowing us to evaluate metabolic response to therapy.
The unprecedented rates of high-quality responses afforded by more effective classes of novel agents and the association between the depth of response and long-term outcomes3 have recently led to refinement of the response criteria by using more sensitive techniques for assessment of minimal residual disease (MRD), both inside and outside the bone marrow (BM).4 More specifically, owing to the patchy pattern of bone marrow plasma cell (BMPC) infiltration and the existence of extramedullary sites of clonal PCs, a new response category of imaging-MRD negativity has been identified, based on the disappearance of every area of increased tumor metabolism assessed with functional imaging techniques.
Almost 10 years ago, the Little Rock group first evaluated the role of FDG PET/CT in the context of the Total Therapy 3, demonstrating that PET-positive lesions at diagnosis and during/after the completion of therapy were predictive of prognosis.5 Several other independent studies confirmed the improved outcomes of patients achieving PET negativity after transplant, including those in conventionally defined complete remission.6 On the basis of these results, FDG PET/CT is actually considered the preferred imaging technique for evaluating and monitoring metabolic response to therapy.7 However, it is important to emphasize that both false-negative and false-positive results may occur with use of FDG PET/CT. In particular, false-negative scans can be related to hyperglycemia or recent administration of high-dose steroids, leading to a transient metabolic suppression. Moreover, it has been reported that, in a variable though not yet well-defined rate of patients, PCs may not be FDG avid.
In addition to FDG, new PET/CT tracers targeting different metabolic pathways or receptors expressed by MM cells, and acting as molecular imaging biomarkers, potentially more sensitive, have been preliminarily investigated in limited series of MM patients; however, their lower availability, the lack of direct comparisons with FDG, and the interpatient tumor heterogeneity regarding specific targets prevent any definite conclusion from being drawn.8,9
Initial experience with functional MRI approaches, such as diffusion weighted imaging (DWI), enabling quantitative assessment of disease burden by quantifying the molecular diffusion of body water and the microcirculation of blood in the capillary network, showed a high sensitivity of the technique, in particular for detection of diffuse BMPC infiltration, a higher correlation with BM trephine samples in comparison with PET/CT, and the capability to identify significant changes in patients in remission after therapy.10 However, published studies were based on a limited number of heterogeneous patients, and no standardization in the interpretation of the results is currently available.
In the study by Rasche et al, 227 newly diagnosed, transplant-eligible, MM patients were prospectively and simultaneously studied at baseline with DWI whole-body MRI and FDG PET, along with fluorescence in situ hybridization and gene expression profiling (GEP). The study was aimed at identifying tumor-intrinsic features associated with PET false negativity and a DWI MRI–positive pattern. They found that the majority of the patients were positive for both imaging techniques (81%), 7% were double negative, and 11% belonged to the PET false-negative/DWI MRI–positive subgroup (see figure). None of these latter patients belonged to the GEP-defined HY or PR molecular subgroup. Most importantly, among 21 differentially expressed genes in comparison with the double-positive imaging group, the top gene was the one coding for HK2, with significantly lower expression in the PET false-negative group, along with deregulation of the other 5 genes involved in metabolism. HK2 is involved in the first step of glycolysis, necessary for the phosphorylation of both normal glucose and FDG; a lower activity of this enzyme results in lower levels of metabolically trapped tracer in tumor cells. Therefore, a mechanistic explanation for PET false negativity was provided for the first time by this study. Unfortunately, the mechanism underlying differential HK2 expression remains elusive. Another caveat is that 15% of the patients in the PET false-negative group showed FDG avid disease at the time of relapse, a finding not yet clarified.
The use of GEP to identify those MM patients for whom PET/CT is not an appropriate tool for assessment of bone disease and/or for evaluation of metabolic response to therapy is an important step toward a more “personalized” and appropriate use of diagnostic and prognostic resources. Which should be the next steps? First of all, homogeneous and prospective comparison of DWI MRI with PET/CT, both prior and after treatment, is needed to optimize the use of imaging for prognosis and for evaluation of metabolic response to therapy. Second, PET/CT interpretation criteria were not homogeneous in different studies; similarly, no consensus exists for DWI MRI. Attempts to standardize FDG PET/CT interpretation criteria are ongoing. Regarding MRD evaluation, it will be important to establish the relationship between complete metabolic response and MRD negativity at the BM level, as well as to define the impact of MRD assessment on treatment strategies. Upcoming prospective trials, extensively applying novel techniques evaluating MRD both inside and outside the BM, will help to address these issues and define the role of these promising tools in clinical practice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal