Abstract
We performed a meta-analysis to evaluate the risk of venous thromboembolism (VTE) in pregnant women with essential thrombocythemia. Twenty-one trials and 756 pregnancies met inclusion criteria. The absolute VTE risk in the antepartum period is not above a threshold where low-molecular-weight heparin (LMWH) prophylaxis is clearly indicated or below a threshold where LMWH should be withheld (2.5%; 95% CI, 1.3-4.3). Postpartum, the absolute VTE risk is above a threshold where postpartum LMWH prophylaxis should be considered (4.4%; 95% CI, 1.2-9.5).
Case presentation
A 28-year-old woman with JAK2-positive essential thrombocythemia was referred to your clinic to discuss the potential benefit of low-molecular-weight heparin prophylaxis during a future pregnancy to prevent venous thromboembolism (VTE). She is currently on aspirin and hydroxyurea for a history of problematic erythromyalgia. She has no personal or family history of VTE.
Introduction
Essential thrombocythemia (ET) is a clonal stem cell disorder involving the megakaryocytic lineage that is associated with the JAK2, CALR, and MPL somatic mutations, with 15% to 20% of diagnoses made in women of childbearing age.1,2 Patients with ET present with sustained thrombocytosis ≥450 × 109/L and episodes of venous and/or arterial thrombosis, microcirculatory symptoms or bleeding secondary to acquired von Willebrand disease (VWD).2,3 Published studies in women with ET have primarily focused on the outcomes of live birth rate and pregnancy complications,4-8 with little data available on the absolute risk of VTE (deep vein thrombosis [DVT] and pulmonary embolism [PE]) during pregnancy or the 6-week postpartum period.
The benefit of low-molecular-weight heparin (LMWH) prophylaxis is generally accepted when the absolute risk of VTE during pregnancy or in the postpartum period is >3%, which is based on a net clinical benefit of preventing VTE that outweighs any harm from major bleeding, taking into account both the absolute rate and case fatality rate of thrombosis and bleeding.9-11 Likewise, an absolute VTE risk during pregnancy or postpartum that is <1% would not warrant LMWH prophylaxis.9-11 For reference, the VTE risk among women who are pregnant in the general population is 0.1% in the antepartum period (5-12 per 10 000 pregnancies) and 0.05% in the postpartum period (3-7 per 10 000 deliveries), where the risk of LMWH prophylaxis clearly outweighs the benefits.9,12
The aim of our meta-analysis is to quantify the absolute risk of VTE in women with ET during pregnancy to provide guidance on the use of antepartum and postpartum LMWH prophylaxis.
Methods
Study selection
A systematic literature search was conducted on MEDLINE (1946 to May 2016), EMBASE (1947 to May 2016), and EBM reviews using the Cochrane Database of Systematic Review (2005 to May 2016), ACP Journal Club (1981 to April 2016), Database of Abstracts of Reviews of Effects (1st Quarter 2016), Cochrane Central Register of Controlled Trials (April 2016), Cochrane Methodology Register (3rd Quarter 2012), Health Technology Assessment (2nd Quarter 2016), and National Health Service Economic Evaluation (1st Quarter 2016) using an OVID interface. The systematic search strategy is available in supplemental Appendix 1, available on the Blood Web site. References of included studies and narrative reviews were reviewed for additional studies. The last search was completed on 14 May 2016. There was no restriction on date of publication or language (PROSPERO Registration CRD42016039194).
Data extraction and synthesis
Studies were included if they reported outcomes on patients with ET who were pregnant. The primary outcome of the meta-analysis was VTE, defined as DVT, PE, or unusual site thrombosis. Secondary outcomes included bleeding and arterial thromboembolism (ATE) events, including ischemic stroke/transient ischemic attack, myocardial infarction, and arterial embolism. Case series with <5 pregnancies and patients with hereditary thrombocythemia were excluded. Studies were excluded if there was any duplicate reporting over time, with only the most recent study included.
Using a standardized form, data were extracted independently by 2 investigators (L.S. and M.A.R.). Disagreements of study inclusion or data extracted were resolved by consensus. The data extracted included year of publication, number of participants and pregnancies, primary and secondary outcomes, and use of antepartum and postpartum LMWH prophylaxis. Antepartum LMWH prophylaxis use is defined as LMWH use for at least 4 weeks duration during pregnancy. Details of aspirin (ASA) and interferon (IFN) were also recorded. Authors were contacted for data clarification, including confirmation of any patient overlap between any published studies.
Primary and secondary outcomes were reported per pregnancy in the antepartum and postpartum periods. Confidence intervals for proportions were calculated using the random effects model. The VTE and bleeding risk were reported according to LMWH, ASA, and IFN use, when available. Pregnancies ending in first trimester loss (≤12 weeks’ gestation) were excluded a priori from the postpartum VTE analysis. Peripartum bleeding, defined as bleeding from the time of delivery to 24 hours postpartum, was excluded from analysis. Placental abruption was included as bleeding events.
Study quality with respect to LMWH use was assessed using the Newcastle-Ottawa Quality Assessment Scale. Data analysis was completed using StatsDirect version 2.8.0 (StatsDirect Ltd, Cheshire, UK). Our treatment recommendations are based on the quality of available evidence and are outlined using the Grading of Recommendations Assessment Development and Evaluation tool.13
Results
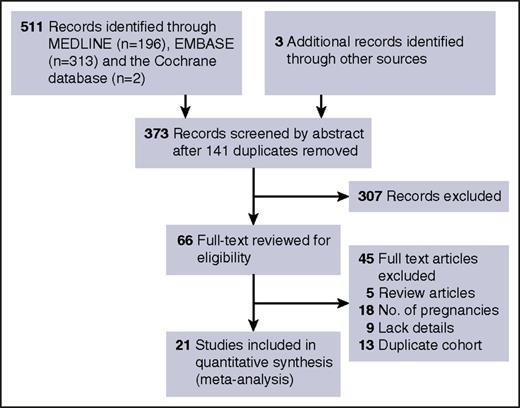
Our search strategy identified 514 records, of which 21 publications including 504 patients and 756 pregnancies met eligibility criteria (Figure 1).4-7,14-31 Seventeen investigators were contacted and 10 provided additional information.
Twelve studies included patients with ET based on established diagnostic criteria, according to the Polycythemia Vera Study Group (n = 8),6,14,17,20-22,26,27,32 World Health Organization criteria (n = 4),7,25-27,33 or other criteria (n = 2).16,23,34 The remaining 10 studies did not specify how the diagnosis of ET was established for the patients included. The live birth rate was 74% among 756 pregnancies, with 19.0% (142/749) ending in first trimester loss. Of the 10 studies that reported patients’ past history, 18 of 315 patients had a previous VTE (5.7%). Treatment during pregnancy was variable: 64.4% (284/441) received ASA, 16.8% (82/489) received antepartum LMWH, 29.5% (96/325) received postpartum LMWH, and 14.8% (38/257) received IFN or pegylated-IFN.
Venous thromboembolism risk
Antepartum VTE risk.
Among 756 pregnancies, there were 8 VTE events during pregnancy (1.3%; 95% confidence interval [CI], 0.6-2.2; I2 3.7%). VTE details are described in Table 1. It appears that VTE risk did not change over time (1991-2016) (Table 1). Of the 18 studies that reported VTE outcomes and antepartum LMWH prophylaxis use, there were 0 antepartum VTE events among 82 pregnancies with LMWH use (0%; 95% CI, 0.0-5.7; I2 0%), and 8 antepartum VTE events among 407 pregnancies without LMWH use (2.5%; 95% CI, 1.3-4.3; I2 0%) (Table 2).4-7,14-23,25,28-31
Study characteristics
| Reference . | Year . | Design . | No. of patients . | No. of pregnancies . | VTE . | VTE details . |
|---|---|---|---|---|---|---|
| 14 | 1991 | Retrospective cohort | 6 | 9 | 1 | Antepartum: SVT |
| 15 | 1991 | Retrospective cohort | 8 | 10 | 3 | Postpartum: 1 DVT, 2 PVT |
| 16 | 1994 | Retrospective cohort | 6 | 6 | 0 | — |
| 17 | 1994 | Retrospective cohort | 4 | 7 | 0 | — |
| 18 | 1995 | Retrospective cohort | 4 | 6 | 1 | Postpartum: SVT |
| 19 | 1996 | Retrospective cohort | 10 | 13 | 0 | — |
| 20 | 1996 | Retrospective cohort | 9 | 15 | 1 | Antepartum: PVT/SMV |
| 21 | 2000 | Retrospective cohort | 9 | 17 | 0 | — |
| 22 | 2000 | Retrospective cohort | 12 | 30 | 1 | Antepartum: PE |
| 6 | 2004 | Retrospective cohort | 16 | 40 | 0 | — |
| 7 | 2008 | Retrospective cohort | 36 | 63 | 0 | — |
| 4 | 2009 | Registry | 92 | 122 | 5 | Antepartum: 5 DVT |
| 23 | 2010 | Retrospective cohort | 5 | 5 | 0 | — |
| 24 | 2011 | Retrospective cohort | 13 | 15 | 0 | — |
| 25 | 2011 | Retrospective cohort | 13 | 18 | 0 | — |
| 26 | 2012 | Retrospective cohort | 6 | 15 | 0 | — |
| 27 | 2014 | Retrospective cohort | 158 | 237 | 2 | Postpartum: 1 Budd Chiari, 1 CVT |
| 28 | 2014 | Retrospective cohort | 4 | 9 | 1 | Postpartum: 1 CVT/PE |
| 29 | 2015 | Prospective cohort | 47 | 47 | 0 | — |
| 30 | 2015 | Retrospective cohort | 38 | 62 | 1 | Postpartum: CVT |
| 31 | 2016 | Retrospective cohort | 8 | 10 | 0 | — |
| Reference . | Year . | Design . | No. of patients . | No. of pregnancies . | VTE . | VTE details . |
|---|---|---|---|---|---|---|
| 14 | 1991 | Retrospective cohort | 6 | 9 | 1 | Antepartum: SVT |
| 15 | 1991 | Retrospective cohort | 8 | 10 | 3 | Postpartum: 1 DVT, 2 PVT |
| 16 | 1994 | Retrospective cohort | 6 | 6 | 0 | — |
| 17 | 1994 | Retrospective cohort | 4 | 7 | 0 | — |
| 18 | 1995 | Retrospective cohort | 4 | 6 | 1 | Postpartum: SVT |
| 19 | 1996 | Retrospective cohort | 10 | 13 | 0 | — |
| 20 | 1996 | Retrospective cohort | 9 | 15 | 1 | Antepartum: PVT/SMV |
| 21 | 2000 | Retrospective cohort | 9 | 17 | 0 | — |
| 22 | 2000 | Retrospective cohort | 12 | 30 | 1 | Antepartum: PE |
| 6 | 2004 | Retrospective cohort | 16 | 40 | 0 | — |
| 7 | 2008 | Retrospective cohort | 36 | 63 | 0 | — |
| 4 | 2009 | Registry | 92 | 122 | 5 | Antepartum: 5 DVT |
| 23 | 2010 | Retrospective cohort | 5 | 5 | 0 | — |
| 24 | 2011 | Retrospective cohort | 13 | 15 | 0 | — |
| 25 | 2011 | Retrospective cohort | 13 | 18 | 0 | — |
| 26 | 2012 | Retrospective cohort | 6 | 15 | 0 | — |
| 27 | 2014 | Retrospective cohort | 158 | 237 | 2 | Postpartum: 1 Budd Chiari, 1 CVT |
| 28 | 2014 | Retrospective cohort | 4 | 9 | 1 | Postpartum: 1 CVT/PE |
| 29 | 2015 | Prospective cohort | 47 | 47 | 0 | — |
| 30 | 2015 | Retrospective cohort | 38 | 62 | 1 | Postpartum: CVT |
| 31 | 2016 | Retrospective cohort | 8 | 10 | 0 | — |
CVT, cerebral vein thrombosis; PVT, portal vein thrombosis; SMV, superior mesenteric vein thrombosis; SVT, superficial vein thrombosis.
Proportion of VTE and bleeding in pregnant women with essential thrombocythemia
| . | No LMWH . | LMWH* . |
|---|---|---|
| Antepartum period | ||
| VTE risk (%) | 2.5 (95% CI, 1.3-4.3; I2 0%) (n = 407) | 0.0 (95% CI, 0.0-5.7; I2 0%) (n = 82) |
| Bleeding risk† (%) | 4.0 (95% CI, 1.5-7.8; I2 53.8%) (n = 407) | 0.0 (95% CI, 0.0-5.7; I2 0%) (n = 82) |
| Postpartum period | ||
| VTE risk‡ (%) | 4.4 (95% CI, 1.2-9.5; I2 48%) (n = 229) | 0.0 (95% CI, 0.0-4.6; I2 0%) (n = 96) |
| Bleeding risk† (%) | 2.9 (95% CI, 1.4-5.0; I2 0%) (n = 309) | 2.9 (95% CI, 0.1-9.6; I2 0.1%) (n = 97) |
| . | No LMWH . | LMWH* . |
|---|---|---|
| Antepartum period | ||
| VTE risk (%) | 2.5 (95% CI, 1.3-4.3; I2 0%) (n = 407) | 0.0 (95% CI, 0.0-5.7; I2 0%) (n = 82) |
| Bleeding risk† (%) | 4.0 (95% CI, 1.5-7.8; I2 53.8%) (n = 407) | 0.0 (95% CI, 0.0-5.7; I2 0%) (n = 82) |
| Postpartum period | ||
| VTE risk‡ (%) | 4.4 (95% CI, 1.2-9.5; I2 48%) (n = 229) | 0.0 (95% CI, 0.0-4.6; I2 0%) (n = 96) |
| Bleeding risk† (%) | 2.9 (95% CI, 1.4-5.0; I2 0%) (n = 309) | 2.9 (95% CI, 0.1-9.6; I2 0.1%) (n = 97) |
LMWH use in the antepartum setting is defined as 4 weeks or more of LMWH use during pregnancy.
Including placental abruption.
Excluding first trimester losses.
Among the 18 studies that reported VTE outcomes and ASA use during pregnancy, there were 6 antepartum VTE events among 283 pregnancies with ASA use (2.9%; 95% CI, 1.3-5.1; I2 0%), and 2 antepartum VTE events among 158 pregnancies without ASA use (2.8%; 95% CI, 0.9-5.8; I2 0%). Of the subgroup of 212 pregnancies reported in which ASA was used alone without LMWH, there were 6 antepartum VTE events (4.2%; 95% CI, 2.0-7.1; I2 0%). In comparison, of the 71 pregnancies reported where LMWH and ASA were used, there were 0 antepartum VTE events (0%; 95% CI, 0.0-6.2; I2 0%).
Among the 16 studies that reported VTE outcomes and IFN use during pregnancy, there were 0 antepartum VTE events among 38 pregnancies with IFN use (0%; 95% CI, 0.0-11.9; I2 0%), and 3 antepartum VTE events among 266 pregnancies without IFN use (2.0%; 95% CI, 0.7-3.9; I2 0%).6,14-23,25,28-31 Of the 38 pregnancies with IFN use: 14 received LMWH, 14 received no LMWH, and 10 had unknown LMWH use. Of the 266 pregnancies without IFN: 30 received LMWH, 156 received no LMWH, and 80 had unknown LMWH use. Three episodes of antepartum VTE that occurred in pregnancies without IFN use also received no LMWH prophylaxis.14,20,22
Postpartum VTE risk.
Among 575 pregnancies with postpartum follow-up (excluding first trimester losses), there were 8 VTE events in the postpartum period (1.8%; 95% CI, 0.7-3.4; I2 18.7%) (Table 1).4,6,7,14,15,18-21,23-31 Of the 14 studies that reported VTE outcomes and postpartum LMWH prophylaxis use, there were 0 postpartum VTE events among 96 pregnancies with LMWH use (0%; 95% CI, 0.0-4.6; I2 0%) and 6 postpartum VTE events among 229 pregnancies without LMWH use (4.4%; 95% CI, 1.2-9.5; I2 48%) (Table 2).4,6,7,14,15,18-21,23,25,28,30,31 Insufficient detail was available with respect to IFN or ASA use and postpartum VTE.
Bleeding risk.
There were 12 antepartum bleeds reported in 756 pregnancies (2.3%; 95% CI, 0.9-4.4; I2 46.9%), comprised of 9 placental abruptions, 2 epistaxis, and 1 vaginal bleeding in the first trimester of pregnancy. There were 11 postpartum bleeds reported in 575 pregnancies with postpartum follow-up (2.3%; 95% 0.9-4.3; I2 41.3%),4,6,7,14,15,18-21,23-31 comprised of 7 postpartum hemorrhages, 2 hematomas post–cesarean section, and 2 episodes of bleeding after pregnancy loss. Bleeding outcomes with and without LMWH prophylaxis in the antepartum and postpartum periods are presented in Table 2.
Among the 17 studies that reported bleeding outcomes and ASA use during pregnancy, there were 6 antepartum bleeding events among 191 pregnancies with ASA use (4.3%; 95% CI, 1.4-8.7; I2 30.5%) and 4 antepartum bleeding events among 129 pregnancies without ASA use (4.9%; 95% CI, 2.0-9.0; I2 0%). Of the subgroup of 119 pregnancies reported in which ASA was used alone without LMWH, there were 6 antepartum bleeding events (6.7%; 95% CI, 2.3-13.2; I2 32.5%). In comparison, of the 71 pregnancies reported in which LMWH and ASA were used, there were 0 antepartum bleeding events (0%; 95% CI, 0.0-6.2; I2 0%). Insufficient detail was available with respect to ASA use and postpartum bleeding.
Arterial thromboembolism risk.
Among 756 pregnancies, there were 4 possible ATE events described in the antepartum period: transient visual loss after ASA was held for 1 week, an ocular transient ischemic attack at 6 weeks’ gestation while on ASA, and 2 patients who described both visual disturbance and dizziness with unknown ASA use.6,21,30 No patient with possible ATE received LMWH prophylaxis. No details were available with respect to IFN use and possible ATE risk.
Study quality
There was representative patient selection, but the comparability of cohorts and adequacy of follow-up was limited in all studies because of retrospective study design and inconsistent reporting. Details of study quality are further outlined in Table 3 using the Newcastle-Ottawa Quality Assessment Scale.
Study quality based on the Newcastle-Ottawa Quality Assessment Scale
| Reference . | 14 . | 15 . | 16 . | 17 . | 18 . | 19 . | 20 . | 21 . | 22 . | 6 . | 7 . | 4 . | 23 . | 24 . | 25 . | 26 . | 27 . | 28 . | 29 . | 30 . | 31 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | |||||||||||||||||||||
| Representativeness of exposed (LMWH) cohort | • | • | • | N/A | N/A | N/A | • | • | • | N/A | N/A | • | N/A | • | • | • | N/A | N/A | • | • | • |
| Selection of the nonexposed (no LMWH) cohort | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | N/A | • | • | • | • |
| Ascertainment of exposure | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | — | — | • | • | • | • |
| Outcome not present at beginning of study | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Comparability | |||||||||||||||||||||
| Comparability of cohorts | — | — | — | N/A | N/A | N/A | — | — | — | N/A | N/A | — | N/A | — | — | — | N/A | N/A | — | — | — |
| Outcome | |||||||||||||||||||||
| Assessment of outcome | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Was follow-up long enough? | • | • | — | — | • | • | • | • | — | • | • | • | • | • | • | • | • | • | • | • | • |
| Adequacy of follow-up | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Reference . | 14 . | 15 . | 16 . | 17 . | 18 . | 19 . | 20 . | 21 . | 22 . | 6 . | 7 . | 4 . | 23 . | 24 . | 25 . | 26 . | 27 . | 28 . | 29 . | 30 . | 31 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | |||||||||||||||||||||
| Representativeness of exposed (LMWH) cohort | • | • | • | N/A | N/A | N/A | • | • | • | N/A | N/A | • | N/A | • | • | • | N/A | N/A | • | • | • |
| Selection of the nonexposed (no LMWH) cohort | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | N/A | • | • | • | • |
| Ascertainment of exposure | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | — | — | • | • | • | • |
| Outcome not present at beginning of study | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Comparability | |||||||||||||||||||||
| Comparability of cohorts | — | — | — | N/A | N/A | N/A | — | — | — | N/A | N/A | — | N/A | — | — | — | N/A | N/A | — | — | — |
| Outcome | |||||||||||||||||||||
| Assessment of outcome | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Was follow-up long enough? | • | • | — | — | • | • | • | • | — | • | • | • | • | • | • | • | • | • | • | • | • |
| Adequacy of follow-up | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
Discussion
Based on our meta-analysis of 504 women with ET who had 756 pregnancies, there is an increased absolute risk of VTE compared with that for the general pregnant population. This is the largest meta-analysis published to date that quantifies VTE and bleeding risk in pregnant women with ET. Our results are strengthened by the additional data provided by 10 of the investigators.
Among the subgroup of pregnancies in which LMWH was not given, the absolute risk of VTE was 2.5% (95% CI, 1.3-4.3) in the antepartum period and 4.4% (95% CI, 1.2-9.5) in the postpartum period. Based on a previously defined absolute VTE risk threshold (>3%) for LMWH use in pregnancy, we suggest LMWH prophylaxis in the postpartum period. However, we also acknowledge that the lower bound of the confidence interval is between 1% to 3%, so taking into account patient preferences and values and the patient’s bleeding risk is still needed. The absolute VTE risk in the antepartum period is between 1% and 3%, so the risk-benefit balance for antepartum LMWH prophylaxis use is not clear.9,10 Of note, the upper bound of the confidence interval of VTE risk in the antepartum period is 4.3%, which is above the threshold (>3%) to recommend antepartum LMWH prophylaxis. These results highlight the need for further research to provide more precise estimates of absolute VTE risk.
Individualized and shared decision-making is important in scenarios where clinical equipoise exists.12 Downsides of LMWH include burden of injections (up to 400 injections/pregnancy), cost (>$4000/pregnancy), reduced likelihood of epidural anesthesia and side effects of LMWH such as major bleeding, and, rarely, heparin induced thrombocytopenia or osteoporotic fracture.35-38 Because of the drawbacks of LMWH, some patients may only choose LMWH prophylaxis for the 6-week postpartum period, with clinical vigilance in the antepartum period. If preventing venous thrombosis is of high value, patients may choose both antepartum and postpartum prophylaxis.
Additional risk factors for thrombosis and bleeding should also be taken into account. Specifically, women with ET and a past history of DVT, PE, or unusual site thrombosis warrant prophylactic or higher doses of LMWH in the antepartum and postpartum periods. Although this group was not well represented in our study, extrapolated data from non-ET pregnant patients supports use of LMWH prophylaxis in pregnancy for all women with previous unprovoked or estrogen-associated VTE, and possibly for those with a history of provoked VTE in the setting of thrombophilia.12,39 There are no data available in women with ET and transient thrombotic risk factors during pregnancy,12,39 or risk factors specific to patients with ET. The IP-SET thrombosis prognostic score (age >60, thrombosis history, cardiovascular risk factors, JAK-2 status) has not been studied in pregnancy, and many of the risk factors are not relevant to young pregnant women.40 Cardiovascular risk factors (smoking, hypertension, or diabetes) and elevated white blood cell count are associated with thrombosis in nonpregnant ET patients,40,41 and ET patients with a JAK2 mutation have a higher rate of thrombotic events in the nonpregnant population when compared with those with a CALR mutation.42,43 Further risk stratification is needed in pregnant women with ET to better determine their risk of thrombosis.
Women with ET may also have an increased risk of bleeding because they are often on antiplatelet therapy or may develop acquired VWD as a result of thrombocytosis. Although bleeding risk was low in our meta-analysis, 2 women with acquired VWD had major bleeding despite no LMWH or ASA use.21 In a retrospective study evaluating nonpregnant patients, patients with the CALR mutation had excessive bleeding and no reduction in thrombotic risk with ASA for primary prevention.44 It is reassuring that the number of bleeding events in the antepartum and postpartum periods is comparable or lower with the total bleeding rates reported in randomized trials evaluating prophylactic LMWH and ASA during pregnancy or in the postpartum period in women without ET.45-48 It is further reassurance that there was no antepartum bleeding reported in women with ET who received a combination of ASA and LMWH during their pregnancies.
There are several limitations to our meta-analysis. All but one study was retrospective in nature. There is the possibility of reporting or ascertainment bias because the thrombosis or bleeding events were not the primary outcome of interest in the included studies so events may not have been systematically or accurately captured. The choice of LMWH use was not randomized; patients who were deemed at higher thrombotic risk may have received LMWH, which could have affected the estimated risk of VTE. There were no details available on how women with past VTE (5.7% of the population studied) were treated in subsequent pregnancies. There were also no platelet count values or von Willebrand factor levels available to correlate with VTE or bleeding outcomes.
We chose to exclude first trimester losses a priori when assessing postpartum VTE risk, because the postpartum risk of thrombosis is presumably lower after a first trimester loss compared with late pregnancy loss or full-term pregnancy. On review of our data, there were no thrombotic events that occurred after a first trimester loss; we would not have missed any of the VTE events because of our a priori exclusion.
Given that the included studies spanned over more than 2 decades (1991-2016), there may have been changes over time to the definition of ET, in study reporting or in obstetrical management that may have indirectly affected reporting of VTE and bleeding events. On the contrary, it appears thrombotic and bleeding events occurred in a similar rate across time. Finally, the VTE or bleeding events recorded were not based on standardized definitions or were independently adjudicated, limiting data interpretation. There was no detail available on whether the VTE events reported were objectively confirmed, including if DVTs involved the proximal or distal vein segments, or if PEs involved the segmental or subsegmental arteries. There was also not enough information to extract out major bleeding events from non–major bleeding events according to standardized International Society on Thrombosis and Haemostasis definitions.49,50 A prospective registry is ongoing and may provide additional data on VTE and bleeding risk in women with ET and pregnancy.51
Although LMWH reduces the risk of thrombosis, it does not affect the underlying disease or platelet count. There were only a small proportion of pregnancies where IFN was given, limiting conclusions on the role of disease-controlling therapy to prevent thrombosis. The use of ASA in patients with ET has primarily been extrapolated from the ECLAP randomized controlled trial data evaluating ASA vs placebo in patients with polycythemia vera.52 In patients without myeloproliferative disorders, ASA has been found to have a modest VTE risk reduction in patients with a single previous unprovoked VTE not requiring anticoagulation; however, how this relates to pregnancy or patients with ET is unclear.53-55 The role of ASA for primary and secondary VTE prevention and bleeding risk still needs to be defined in ET and pregnancy. ASA may provide additional protection against uncommon but serious ATE events. The role of ASA in preventing future pregnancy loss is controversial, with mixed results based on retrospective studies and registries.4-8,21,22
In women with ET, there is an increased risk of pregnancy loss based on retrospective cohort studies and registry data, with a variable risk published of placenta-mediated pregnancy complications such as preeclampsia or placental abruption.4-8 Of note, we found a higher number of pregnancies ending in placental abruption than expected. Prospective studies or a meta-analysis is still needed to better quantify the risk, if any, of placenta-mediated pregnancy complications in women with ET, including what role ASA, LMWH, or IFN has in preventing pregnancy loss or placenta-mediated complications. In the non-ET population, recent data from randomized controlled trials and an individual patient-level meta-analysis of 963 pregnant women in 8 trials found that there is no role for antepartum ASA or LMWH prophylaxis in preventing pregnancy loss or placenta-mediated pregnancy complications, including those with inherited thrombophilia,48,56-60 with the exception of ASA and preeclampsia.60 Further research evaluating the role of LMWH for the subgroup of women with past placenta abruption is still needed.59
In summary, the absolute venous thrombotic risk is increased to 1% to 3% during the antepartum period and >3% in the postpartum period among women with ET. LMWH prophylaxis is suggested in the postpartum period. The role of thromboprophylaxis requires a shared decision-making process, taking into account patient values and preferences, the risk of bleeding, and the risks and benefits of LMWH. Future prospective studies of pregnant patients with myeloproliferative disorders are still needed to better quantify thrombotic and bleeding events, and the use of standardized definitions of VTE and bleeding with independent event adjudication should be encouraged.49,50
Recommendations
In pregnant women with ET, antepartum vigilance is recommended. LMWH prophylaxis should be considered based on the presence of additional risk factors and a preferences- and values-based discussion, given a modest absolute risk of VTE (Grade 2C, weak recommendation with low-quality evidence).
In women with ET during the postpartum period, we suggest the use of LMWH prophylaxis to prevent thrombosis over no LMWH based on an important absolute risk of VTE (Grade 2C, weak recommendation with low-quality evidence).
Case resolution
We had a detailed discussion with the patient about the risks and benefits of LMWH in the antepartum and postpartum period, including reviewing the absolute VTE and bleeding risks. We discussed the potential for increased bleeding with the combination of LMWH prophylaxis and ASA use. She decided to continue low-dose ASA in her pregnancy and declined antepartum LMWH prophylaxis. The patient accepted our recommendation for 6 weeks of LMWH prophylaxis in the postpartum period.
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge and thank the following investigators for providing additional data or clarifications: S. Alimam (Department of Haematology, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom), S. Betti (Institute of Hematology, Catholic University, Rome, Italy), R. Ciancia (Division of Hematology, National Cancer Institute, Aviano, Italy), R. Cincotta (Department of Maternal Fetal Medicine, Mater Mothers' Hospital, South Brisbane, Queensland, Australia), V. De Stefano (Institute of Hematology, Catholic University, Rome, Italy), N. Gagnat (Division of Hematology, Mayo Clinic, Rochester, MN), F. Giona (Department of Cellular Biotechnologies and Haematology, Sapienza University, Rome, Italy), L. Melillo (Division of Hematology, IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy), F. Palandri (Department of Hematology/Oncology, Seràgnoli Institute of Hematology, Bologna University School of Medicine, Bologna, Italy), F. Passamonti (Division of Hematology, University of Insurbia, Varese, Italy), M. L. Randi (Department of Medicine, University of Padua, Padua, Italy), S. Robinson (Department of Haematology, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom), F. Rodeghiero (Department of Cell Therapy and Hematology, San Bortolo Hospital, Vincenza, Italy), and E. Rumi (Department of Oncology and Hematology, Università di Pavia, Pavia, Italy).
The study was supported by a CanVECTOR Research Fellowship award (L.S.); a Heart and Stroke Foundation New Investigator Award and a University of Ottawa Faculty of Medicine Clinical Research Chair in Venous Thromboembolism and Cancer (M.C.); and a Heart and Stroke Foundation Career Investigator Award (CI6225 and CI7441) and a University of Ottawa Faculty of Medicine Clinical Research Chair in Venous Thrombosis and Thrombophilia (M.A.R.).
Authorship
Contribution: M.A.R. had the initial idea for the study; L.S. and M.A.R. developed the methods for the systematic review and meta-analysis, participated in the review and selection of included publications, and participated in the data extraction; M.C. and M.A.R. participated in document translation; L.S. and M.C. participated in the data analysis; S.E.R. and S.A. were investigators for a component study and provided additional insight into study design and data analysis; L.S. wrote the first draft of the manuscript; and all authors reviewed drafts of the manuscript and approved the final draft of the manuscript.
Conflict-of-interest disclosure: M.C. received honoraria from Pfizer and Leo Pharma. S.E.R. received honoraria from Novartis and Pharmacosmos, and grant funding from Amgen and Octapharma. The remaining authors declare no competing financial interests.
Correspondence: Marc A. Rodger, The Ottawa Hospital, Centre for Practice-Changing Research, 501 Smyth Rd, Box 201, Ottawa, ON K1H 8L6, Canada; e-mail: mrodger@ohri.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal