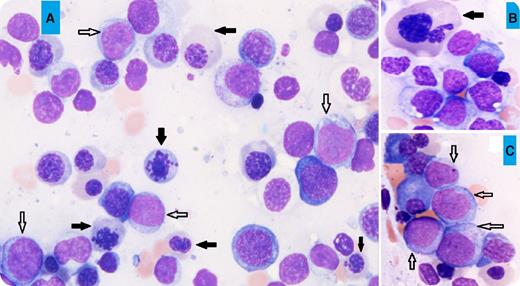
A 69-year-old man presented with increasing fatigue over 3 months. The blood work showed pancytopenia (white blood cell count, 2.6 × 109/L; hemoglobin, 5.3 g/dL; and platelet count, 17 × 109/L). A bone marrow aspirate showed hypercellular marrow with erythroid predominance (60%), trilineage dysplasia including marked dyserythropoiesis (megaloblastoid maturation, karyorrhexis, and nuclear budding; black arrows, panels A-B; for all panels, original magnification ×40, May-Grünwald stain), and 15% blasts of all marrow nucleated cells (white arrows, panels A,C), but 38% blasts of the nonerythroid cells. The flow cytometry study showed that the blasts were positive for CD34, CD117, HLA-DR, CD13, CD33, CD7, and myeloperoxidase, and negative for other lymphoid-associated markers, consistent with myeloid blasts. The conventional cytogenetic analysis revealed a complex abnormal karyotype: 46,XY, del(5)(q13q31), −7, add(11)(p15), −18, −20.
Due to the presence of ≥20% blasts in the nonerythroid cell population, this would be classified as acute erythroid leukemia (AEL; erythroid/myeloid) according to the 2008 World Health Organization (WHO) classification. However, it should now be classified as myelodysplastic syndrome (MDS) with excess blasts-2 based on the 2016 revised WHO classification. Recent studies suggest that the previously named AEL had a closer relationship to MDS than de novo acute myeloid leukemia in terms of morphologic and genetic features.
A 69-year-old man presented with increasing fatigue over 3 months. The blood work showed pancytopenia (white blood cell count, 2.6 × 109/L; hemoglobin, 5.3 g/dL; and platelet count, 17 × 109/L). A bone marrow aspirate showed hypercellular marrow with erythroid predominance (60%), trilineage dysplasia including marked dyserythropoiesis (megaloblastoid maturation, karyorrhexis, and nuclear budding; black arrows, panels A-B; for all panels, original magnification ×40, May-Grünwald stain), and 15% blasts of all marrow nucleated cells (white arrows, panels A,C), but 38% blasts of the nonerythroid cells. The flow cytometry study showed that the blasts were positive for CD34, CD117, HLA-DR, CD13, CD33, CD7, and myeloperoxidase, and negative for other lymphoid-associated markers, consistent with myeloid blasts. The conventional cytogenetic analysis revealed a complex abnormal karyotype: 46,XY, del(5)(q13q31), −7, add(11)(p15), −18, −20.
Due to the presence of ≥20% blasts in the nonerythroid cell population, this would be classified as acute erythroid leukemia (AEL; erythroid/myeloid) according to the 2008 World Health Organization (WHO) classification. However, it should now be classified as myelodysplastic syndrome (MDS) with excess blasts-2 based on the 2016 revised WHO classification. Recent studies suggest that the previously named AEL had a closer relationship to MDS than de novo acute myeloid leukemia in terms of morphologic and genetic features.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal