Key Points
Active MMP-2 enhances platelet activation by cleaving PAR1 at an extracellular site different from the thrombin cleavage site.
The novel PAR1-tethered ligand exposed by MMP-2 selectively stimulates PAR1-dependent Gq and G12/13 pathway activation.
Abstract
Platelets contain and release several matrix metalloproteinases (MMPs). Among these, active MMP-2 enhances platelet aggregation by favoring the activation of phosphatidylinositol 3- kinase (PI3K) and contributes to arterial thrombosis. The platelet surface target of MMP-2 and the mechanism through which it primes platelets to respond to subsequent stimuli are still unknown. We show that active MMP-2 enhances platelet activation induced by weak stimuli by cleaving PAR1 at a noncanonical extracellular site different from the thrombin-cleavage site and thus initiates biased receptor signaling, triggering only some of the signaling pathways normally activated by full PAR1 agonism. The novel PAR1-tethered ligand exposed by MMP-2 stimulates PAR1-dependent Gq and G12/13 pathway activation, triggering p38-MAPK phosphorylation, Ca+2 fluxes, and PI3K activation, but not Gi signaling; this is insufficient to cause platelet aggregation, but it is enough to predispose platelets to fully respond to Gi-activating stimuli. Integrin αIIbβ3 is a necessary cofactor for PAR1 cleavage by MMP-2 by binding the MMP-2 hemopexin domain, thus favoring the interaction of the enzyme with PAR1. Our studies unravel a novel mechanism regulating platelet activation that involves the binding of MMP-2 to integrin αIIbβ3 and the subsequent cleavage of PAR1 by active MMP-2 at a noncanonical site, exposing a previously undescribed tethered ligand that triggers biased G-protein agonism and thus predisposes platelets to full activation by other stimuli. These results identify the MMP-2-αIIbβ3-PAR1 interaction as a potential target for the prevention of arterial thrombosis.
Introduction
Matrix metalloproteinases (MMPs), a family of zinc- and calcium-dependent proteolytic enzymes that act extracellularly at neutral pH, are important regulators of platelet function.1-3 Platelets express several MMPs, including MMP-1, MMP-2, MMP-3, and MMP-14, and some of their tissue inhibitors, including TIMP-1 and TIMP-2.4-8 Among these, active MMP-2 enhances platelet aggregation induced by several stimuli by favoring the activation of phosphatidylinositol 3-kinase (PI3K).9
The potentiating activity of MMP-2 on platelet activation contributes to arterial thrombosis but only marginally to primary hemostasis, as shown by studies in mice in which the gene for MMP-2 had been disrupted. Crosstransfusion experiments and the generation of chimeric mice showed that platelet-released MMP-2 plays a central role in thrombus formation.10
MMP-2 is released by platelets in vivo at a site of platelet plug formation in healthy humans11 and in the coronary artery where a thrombus is forming in patients with acute coronary syndromes, and it contributes to sustain platelet activation.12 Moreover, MMP-2 of carotid artery atherosclerotic plaques promotes platelet activation and participates in the generation of ischemic cerebrovascular events.13
Intracellular MMP-2 has been reported to regulate agonist-induced platelet aggregation via the hydrolytic cleavage of talin, thus facilitating inside-out αIIbβ3 activation.14,15 Moreover, it was recently shown that extracellular pro-MMP-2 binds to activated integrin αIIbβ3 on platelets and is then converted into active MMP-2, an effect competitively antagonized by fibrinogen.16 However, the binding of MMP-2 to αIIbβ3 cannot explain some of the signaling events triggered by MMP-2, such as protein kinase C (PKC) or phospholipase C activation.9 Therefore, the initial platelet-surface target of MMP-2 mediating its priming effect on platelet activation is still unknown.
In order to exert its potentiating activity on platelets, MMP-2 must be enzymatically active; in fact, pro-MMP-2 or heat-inactivated MMP-2 does not potentiate platelet activation.9 It can thus be hypothesized that MMP-2 enzymatically cleaves a G-protein-coupled receptor (GPCR), triggering the dissociation of β/γ heterodimers17 that in turn starts a number of signaling events leading to enhanced αIIbβ3 activation and platelet aggregation.9
Typical GPCRs activated by enzymatic cleavage on platelets are the proteinase-activated receptors (PARs),18,19 and a growing number of proteases besides thrombin have been shown to cleave PARs. PAR1 in particular is cleaved and activated by several MMPs, including MMP-1, MMP-8, and MMP-13.20-25 However, some proteases, such as elastase and activated protein C, trigger only some of the signaling pathways normally activated by full PAR agonists, a phenomenon referred to as biased signaling.26
We show here that active MMP-2 enhances platelet activation and aggregation by cleaving platelet PAR1 at a noncanonical site different from the thrombin or MMP-1 cleavage sites and generates a new tethered ligand that triggers PAR1-biased signaling, which is unable to induce aggregation but primes platelets to full activation by other stimuli. We also show that platelet integrin αIIbβ3 is a necessary cofactor for the activity of MMP-2 on platelets, as it binds the MMP-2 PEX domain and facilitates MMP-2-dependent PAR1 cleavage and, ultimately, platelet activation. These studies unravel a novel mechanism finely tuning platelet activation in response to stimuli and identify potential new targets for antiplatelet therapy.
Methods
Activation of gelatinase A
Recombinant human pro-MMP-2 (R&D Systems, Minneapolis, MN) was activated as described previously.11,27 In some experiments, MMP-2 was heat denatured at 96°C for 5 minutes or blocked with the specific human tissue inhibitor TIMP-2 (Chemicon International, Temecula, CA) (47.5 nM) for 2 minutes or with a blocking anti–human MMP-2 antibody (Millipore, Temecula, CA) (4 µg/mL) for 10 minutes before use.
Preparation of platelets
Cell culture and transfection
Chinese hamster ovary (CHO) cells stably expressing integrin αIIbβ3 were obtained as described elsewhere.30,31 CHO cells were transiently transfected with pcDEF3/PAR1 T7-tagged wild-type (WT) or pcDEF3 coding for T7-tagged PAR1 mutants T37D, L38S, D39S, P40N, R41A, S42D, and F43R using Turbofect (Fermentas, Glen Burnier, MD). All experiments with transiently transfected cells were performed 48 hours after transfection.
Adhesion, spreading, and protein phosphorylation
CHO cells and human platelets were layered on human fibrinogen and adhesion, spreading, and protein phosphorylation were assessed as described in supplemental Methods (available on the Blood Web site).
Flow cytometry
Expression of P-selectin and PAR1 by platelets and CHO cells expressing T7-tagged human PAR1 was assessed by flow cytometry, as described in supplemental Methods.
Platelet PAR1 desensitization and internalization
PAR1 desensitization was obtained by incubating platelets with 100 µM thrombin receptor activating peptide (TRAP)–6 (Bachem, Bubendorf, Switzerland) in the presence of Integrilin (5 µg/mL), an αIIbβ3 blocker, for 30 minutes at room temperature without stirring. After the addition of 5 vol washing buffer, platelets were recovered by centrifugation and resuspended at 300 000/µL in Tyrode’s buffer. Desensitization was confirmed by the inability of platelets to aggregate in response to TRAP-6 (100 µM).32
For PAR1 internalization experiments, GFPs were incubated for 1 hour with bovine thrombin (0.5 U/mL), MMP-2 (50 ng/mL), or MMP-2–generated activating peptide DPR-TRAP (10, 100, 300, and 500 µM). Aliquots (5 µL) were then stained with a phycoerythrin-labeled anti–PAR1 antibody (WEDE15) for 30 minutes, and PAR1 expression was analyzed by flow cytometry.
Generation of cAMP and Ca2+ fluxes
GFPs were preincubated with Iloprost (final concentration, 100 ng/mL) for 2 minutes before addition of TRAP-6 (10-500 µM), MMP-2 (0.75 nM), or DPR-TRAP (10-500 µM), alone or in combination. The reaction was stopped after 5 minutes by centrifugation at 12 000g for 10 seconds, and pellets were immediately lysed by the addition of 0.1 N HCl and then analyzed for their cyclic adenosine monophosphate (cAMP) content by a competition-based assay (cyclic AMP immunoassay; General Electric Healthcare, Little Chalfont, UK). Alternatively, platelets were stimulated with TRAP-6 (10-500 µM), MMP-2 (0.75 nM), or DPR-TRAP (10-500 µM) for 5 minutes, the reaction was stopped as described above, and cAMP content was measured after acetylation with acetic anhydride/triethylamine (1 vol/2 vol).
For kinetics of intracellular Ca2+ mobilization, GFPs diluted in Tyrode’s buffer (25 000/µL) were incubated with fluo-3-acetoxymethyl ester for 30 minutes at 37°C.
After acquisition of basal Ca2+ levels for 20 seconds, the tube was removed to add DPR-TRAP at different concentrations (10-500 μM), in the absence or presence of RWJ-56610 (1 μM), and put back immediately. Flow cytometric analysis was performed with an FC500 (Beckman Coulter, Miami, FL).33
RhoA activation assay
RhoA GTPase activity was determined in freshly prepared mouse platelet lysates by using the Rhotekin-RBD bead pull-down assay kit (Cytoskeleton). Washed mouse platelets were incubated with MMP-2 (1.5 nM) for 5 minutes at 37°C and then lysed with cell lysis buffer, and the lysates were immediately used for the pull-down assay. All subsequent incubation and detection steps followed the instructions provided by the manufacturer.
PAR1 site-directed mutagenesis
Plasmid pcDEF3, coding for human T7-tagged WT PAR1, was kindly given by A. Kuliopulos (Tufts University, Boston, MA) and was used for generating all mutants. PAR1 mutants S42D, D39S, and L38S were generated as described previously.34 Primer sequences used for PAR1 mutants were 5′-ATGGGTACCAGTGATCCCC-3′ (L38S forward) and 5′-GGGGATCACTGGTACCCAT-3′ (L38S reverse), 5′-GTACCTTAAGTCCCCGGTCAT-3′ (D39S forward) and 5′-ATGACCGGGGACTTAAGGTAC-3′ (D39S reverse), and 5′- GATCCCCGGGATTTTCTTCTC-3′ (S42D forward) and 5′- GAGAAGAAAATCCCGGGGATC-3′ (S42D reverse). Successful mutagenesis was verified using the ABIPRISM 3130 Genetic Analyses Sequencer (Applied Biosystems).
Data analysis and statistics
All results are expressed as means ± standard error of the mean (SEM). Multiple groups were compared by the 1-way ANOVA. Comparisons between 2 groups were made using the paired Student’s t test. All calculations were performed with the GraphPad Prism 6.0 program. A P value of < .05 was considered statistically significant.
Results
MMP-2 cleaves the PAR1 N-terminal domain at a noncanonical exosite
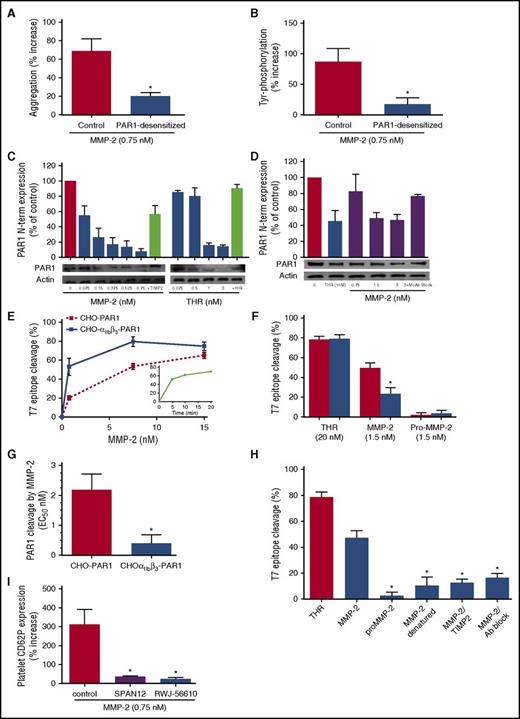
We observed that active MMP-2 was unable to potentiate platelet activation (Figure 1A) or tyrosine phosphorylation (Figure 1B and supplemental Figure 1) of PAR1-desensitized platelets, suggesting a central role of PAR1 for MMP-2 activity on platelets.
MMP-2 cleaves the PAR1 N-terminal domain. (A) Control or PAR1-desensitized platelets were pretreated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of the PAR4 agonist peptide, and aggregation was followed for 5 minutes. Results are expressed as percent increase of aggregation over agonist alone. Data represent means ± SEM (n = 3); *P < .05 vs control. (B) Control or PAR1-desensitized platelets were pretreated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of PAR4 agonist peptide. Tyr phosphorylation was assessed by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs control. (C) Gel-filtered platelets were treated for 10 minutes with thrombin (0.075-3 nM) or MMP-2 (0.075-0.75 nM) in the absence or presence of hirudin (HIR; 3 U/mL) or TIMP-2 (1 µg/mL), respectively, at 37°C. PAR1 N-terminus (N-term) expression was assessed by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3). (D) Platelet-rich plasma was treated for 10 minutes with thrombin (0.1 nM), in the presence of GPRP (2.5 mM) or MMP-2 (0.75-3 nM), in the absence or presence of a monoclonal antibody blocking MMP-2 (MoAb Block), at 37°C. PAR1 N terminus expression was assessed by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3). (E) CHO cells stably expressing αIIbβ3 (CHO-αIIbβ3-PAR1), or not (CHO-PAR1), were transiently transfected with T7-tagged WT PAR1, incubated with increasing concentrations of active MMP-2 for 20 minutes at 37°C, and stained with saturating concentrations of mouse anti-T7 tag. In the inset, CHO cells expressing αIIbβ3 and PAR1 were incubated with MMP-2 (1.5 nM) for increasing periods of time. Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 4). (F) CHO cells stably expressing αIIbβ3 (red columns) or not (blue columns) were transiently transfected with T7-tagged PAR1 and incubated for 20 minutes at 37°C with thrombin (20 nM) or active MMP-2 or pro-MMP-2 (1.5 nM). Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 5); *P < .05 vs CHO-PAR1. (G) EC50 of MMP-2-induced cleavage of PAR1 in CHO cells expressing only PAR1 (CHO PAR1) or both αIIbβ3 and PAR1 (CHO-αIIbβ3-PAR1). Data represent means ± SEM (n = 3); *P < .05 vs CHO- PAR1. (H) CHO-αIIbβ3 cells expressing T7-tagged PAR1 were incubated with thrombin (20 nM), active MMP-2 (1.5 nM), pro-MMP-2, heat-denatured MMP-2, MMP-2 inhibited with TIMP-2 (47.5 nM), or MMP-2 inhibited with a blocking anti–human MMP-2 monoclonal antibody (4 µg/mL) for 20 minutes at 37°C. Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 4); *P < .05 vs MMP-2. (I) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of TRAP-6 (2 µM) in the presence of the monoclonal antibody SPAN12, an immunoglobulin G isotype antibody control (control), or the PAR1 antagonist RWJ-56610 (1 µM). Results are reported as percentage increase CD62P expression vs TRAP-6. Data represent means ± SEM (n = 5); *P < .05 vs control.
MMP-2 cleaves the PAR1 N-terminal domain. (A) Control or PAR1-desensitized platelets were pretreated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of the PAR4 agonist peptide, and aggregation was followed for 5 minutes. Results are expressed as percent increase of aggregation over agonist alone. Data represent means ± SEM (n = 3); *P < .05 vs control. (B) Control or PAR1-desensitized platelets were pretreated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of PAR4 agonist peptide. Tyr phosphorylation was assessed by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs control. (C) Gel-filtered platelets were treated for 10 minutes with thrombin (0.075-3 nM) or MMP-2 (0.075-0.75 nM) in the absence or presence of hirudin (HIR; 3 U/mL) or TIMP-2 (1 µg/mL), respectively, at 37°C. PAR1 N-terminus (N-term) expression was assessed by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3). (D) Platelet-rich plasma was treated for 10 minutes with thrombin (0.1 nM), in the presence of GPRP (2.5 mM) or MMP-2 (0.75-3 nM), in the absence or presence of a monoclonal antibody blocking MMP-2 (MoAb Block), at 37°C. PAR1 N terminus expression was assessed by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3). (E) CHO cells stably expressing αIIbβ3 (CHO-αIIbβ3-PAR1), or not (CHO-PAR1), were transiently transfected with T7-tagged WT PAR1, incubated with increasing concentrations of active MMP-2 for 20 minutes at 37°C, and stained with saturating concentrations of mouse anti-T7 tag. In the inset, CHO cells expressing αIIbβ3 and PAR1 were incubated with MMP-2 (1.5 nM) for increasing periods of time. Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 4). (F) CHO cells stably expressing αIIbβ3 (red columns) or not (blue columns) were transiently transfected with T7-tagged PAR1 and incubated for 20 minutes at 37°C with thrombin (20 nM) or active MMP-2 or pro-MMP-2 (1.5 nM). Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 5); *P < .05 vs CHO-PAR1. (G) EC50 of MMP-2-induced cleavage of PAR1 in CHO cells expressing only PAR1 (CHO PAR1) or both αIIbβ3 and PAR1 (CHO-αIIbβ3-PAR1). Data represent means ± SEM (n = 3); *P < .05 vs CHO- PAR1. (H) CHO-αIIbβ3 cells expressing T7-tagged PAR1 were incubated with thrombin (20 nM), active MMP-2 (1.5 nM), pro-MMP-2, heat-denatured MMP-2, MMP-2 inhibited with TIMP-2 (47.5 nM), or MMP-2 inhibited with a blocking anti–human MMP-2 monoclonal antibody (4 µg/mL) for 20 minutes at 37°C. Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 4); *P < .05 vs MMP-2. (I) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of TRAP-6 (2 µM) in the presence of the monoclonal antibody SPAN12, an immunoglobulin G isotype antibody control (control), or the PAR1 antagonist RWJ-56610 (1 µM). Results are reported as percentage increase CD62P expression vs TRAP-6. Data represent means ± SEM (n = 5); *P < .05 vs control.
Serine proteases, such as thrombin, plasmin, and activated protein C, hydrolyze PAR1 at LDPR41 ↓S42FL (P4P3P2P1↓P1′P2′P3′) to generate the S42FLLRN-tethered ligand (TRAP) that, in turn, activates PAR1 by interacting with the body of the receptor thus triggering transmembrane signaling.35-39 Several MMPs (MMP-1 and MMP-13) also cleave PAR1, but at sites distinct from the thrombin proteolytic site.23,24 Therefore, we assessed the cleavage of platelet PAR1 by MMP-2.
MMP-2 dose-dependently reduced the expression of the platelet PAR1 N-terminal domain (50% effective concentration [EC50] = 0.153 ± 0.019 nM), both in GFPs (Figure 1C) and in plasma (Figure 1D), an effect blocked by TIMP-2 (47.5 nM) or by a monoclonal antibody blocking MMP-2 (4 µg/mL). Concentration-dependent cleavage of PAR1 by active MMP-2 was confirmed in CHO cells expressing T7-tagged PAR1, although it required much higher concentrations (EC50 = 2.18 ± 0.32 nM).
Considering this discrepancy, and in view of previous data of MMP-2-binding to αIIbβ3,16 we performed experiments with CHO cells expressing both T7-tagged PAR1 and integrin αIIbβ3. Cleavage of the PAR1 N terminus by MMP-2 was enhanced in the presence of αIIbβ3 to a level comparable to that observed in platelets (EC50 from 2.18 ± 0.32 nM to 0.37 ± 0.27 nM), while cleavage of PAR1 by thrombin did not vary between the 2 conditions (Figure 1E-G and supplemental Figure 2A). MMP-1 also cleaved the N-terminal domain of PAR1 in CHO cells expressing both PAR1 and αIIbβ3 (supplemental Figure 2B), confirming previous data,23 although less potently than MMP-2 (EC50= 2.13 ± 1.12 nM). PAR1 cleavage by MMP-2 was fast, being almost maximal after only 5 minutes (Figure 1E), and depended on its catalytic activity; in fact, pro-MMP-2, heat-denatured-MMP-2, TIMP-2-blocked MMP-2 or MMP-2 incubated with a blocking monoclonal antibody did not cleave PAR1 (Figure 1H).
The blockade of PAR1 cleavage by an antibody directed against the PAR1 N-terminal residues 35 to 46 (SPAN12), as well as by the PAR1 antagonist RWJ-56610, abolished the potentiating effect of MMP-2 on platelet P-selectin expression (Figure 1I and supplemental Figure 2C). On the contrary, the PAR1 blockers vorapaxar (SCH530348) and SCH79797, which bind PAR1 at a site very close to the extracellular surface,40 did not affect the priming activity of MMP-2 on platelets (supplemental Figure 3A).
Human platelets also express PAR4; thus, we assessed whether it is involved in the activity of MMP-2 on platelets. The selective PAR4 inhibitor YD-3 did not affect the potentiation of tyrosine phosphorylation and of platelet aggregation induced by MMP-2 (supplemental Figure 3B). The persistence of MMP-2 priming effect in platelets in the presence of YD-3 provides evidence that PAR4 is not involved in MMP-2 activity.
Altogether, these data show that MMP-2 potentiates platelet activation by cleaving PAR1 and that the cleavage site is localized between residues 35 and 46 of the N terminus.41 Therefore, in order to identify the specific MMP-2 cleavage site, we performed site-directed mutagenesis of the critical residues between 37 and 43 of the PAR1 N terminus, generating 7 different mutated receptors. We expressed them in CHO cells coexpressing αIIbβ3, and we assessed whether these affect PAR1 cleavage. As a control, we mutated the PAR1 thrombin cleavage site P1′ 42 from serine to aspartate (S42D PAR1), confirming that this mutation suppresses cleavage by thrombin.23
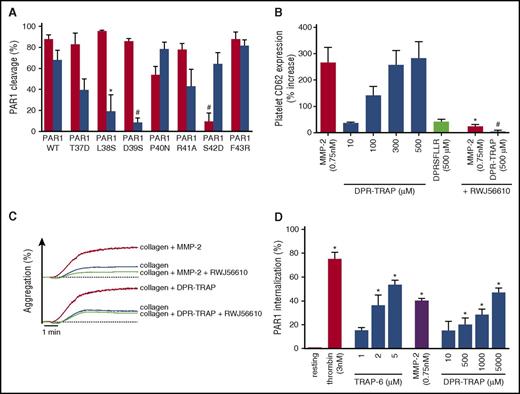
Cleavage of PAR1 by MMP-2 was almost totally suppressed in T7-D39S PAR1-trasfected cells and strikingly reduced in T7-L38S PAR1-transfected cells compared with T7-WT PAR1- or T7-S42D PAR1-transfected cells (Figure 2A). Thus, MMP-2 cleaves PAR1 at TL38↓D39PR, generating a tethered ligand (39DPRSFLLRN) longer than that produced by thrombin.
A MMP-2-generated tethered ligand (DPR-TRAP) potentiates human platelet activation. (A) CHO-αIIbβ3 cells transiently transfected with T7-tagged wild type (WT), T37D, L38S, D39S, P40N, R41A, S42D, and F43R PAR1 were incubated for 20 minutes at 37°C with thrombin (20 nM) (red columns) or active MMP-2 (1.5 nM) (blue columns). Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 5); *P < .05; #P < .005 vs WT. (B) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM), DPR-TRAP (10-500 µM), or DPRSFLLR (500 µM) for 5 minutes and then activated with a subthreshold dose of adenosine 5′-diphosphate (ADP). Preincubation with 1 µM RWJ-56610 together with active MMP-2 (0.75 nM) or DPR-TRAP (500 µM) was also tested. Results are reported as percent increase in CD62P expression vs ADP. Data represent means ± SEM (n = 4); *P < .05 vs MMP-2; #P < .05 vs DPR-TRAP (500 µM). (C) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) or DPR-TRAP (10-500 µM) with or without 1 µM RWJ-56610 and then activated with a subthreshold dose of collagen. Aggregation tracings are representative of 3 experiments. (D) Gel-filtered platelets were incubated with MMP-2 (0.75 nM), thrombin (3 nM), TRAP-6 (1-5 µM), or DPR-TRAP (10-5000 µM) for 1 hour at 37°C. PAR1 internalization was assessed by flow cytometry using the phycoerythrin-WEDE15 antibody. Data represent means ± SEM (n = 3); *P < .05 vs resting.
A MMP-2-generated tethered ligand (DPR-TRAP) potentiates human platelet activation. (A) CHO-αIIbβ3 cells transiently transfected with T7-tagged wild type (WT), T37D, L38S, D39S, P40N, R41A, S42D, and F43R PAR1 were incubated for 20 minutes at 37°C with thrombin (20 nM) (red columns) or active MMP-2 (1.5 nM) (blue columns). Loss of T7 epitope was assessed by flow cytometry. Data represent means ± SEM (n = 5); *P < .05; #P < .005 vs WT. (B) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM), DPR-TRAP (10-500 µM), or DPRSFLLR (500 µM) for 5 minutes and then activated with a subthreshold dose of adenosine 5′-diphosphate (ADP). Preincubation with 1 µM RWJ-56610 together with active MMP-2 (0.75 nM) or DPR-TRAP (500 µM) was also tested. Results are reported as percent increase in CD62P expression vs ADP. Data represent means ± SEM (n = 4); *P < .05 vs MMP-2; #P < .05 vs DPR-TRAP (500 µM). (C) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) or DPR-TRAP (10-500 µM) with or without 1 µM RWJ-56610 and then activated with a subthreshold dose of collagen. Aggregation tracings are representative of 3 experiments. (D) Gel-filtered platelets were incubated with MMP-2 (0.75 nM), thrombin (3 nM), TRAP-6 (1-5 µM), or DPR-TRAP (10-5000 µM) for 1 hour at 37°C. PAR1 internalization was assessed by flow cytometry using the phycoerythrin-WEDE15 antibody. Data represent means ± SEM (n = 3); *P < .05 vs resting.
To assess whether the MMP-2-generated tethered ligand activates PAR1, thus facilitating platelet activation, we synthetized the nona-peptide DPRSFLLRN (DPR-TRAP). DPR-TRAP did not trigger platelet activation, but it enhanced P-selectin expression and platelet aggregation (Figure 2C) induced by several agonists, likewise MMP-2 (Figure 2B and supplemental Figure 3C). We also tested the octapeptide DPRSFLLR for its priming activity on platelets, but this had no activity (Figure 2B). The priming effect of DPR-TRAP was inhibited by the PAR1 antagonist RWJ-56610 (Figure 2B-C).
Moreover, incubation of platelets with DPR-TRAP as well as with MMP-2 induced PAR1 internalization dose dependently (Figure 2D).
MMP-2 interacts allosterically with αIIbβ3
In order to explore the interaction between MMP-2 and αIIbβ3, we performed spreading experiments with CHO cells expressing αIIbβ3 on a fibrinogen-coated surface. Active MMP-2 (0.75 nM) inhibited spreading and focal adhesion kinase (FAK) phosphorylation (Figure 3A). Heat-denatured MMP-2 and MMP-2 blocked by TIMP-2 (47.5 nM) also inhibited spreading on fibrinogen, while pro-MMP-2 did not affect it (Figure 3B). These data show that the interaction between MMP-2 and αIIbβ3 is not dependent on catalytic activity but requires an active molecular conformation of MMP-2. In fact, upon activation, the hemopexin-like domain of MMP-2 undergoes remodeling, becoming more accessible to ligands.42
Effect of MMP-2 on the spreading and FAK phosphorylation of αIIbβ3-expressing CHO cells and platelets. (A) CHO-αIIbβ3 cells were pretreated with active MMP-2 (0.75 nM) (blue columns) or vehicle (red columns) for 5 minutes and then layered on a fibrinogen-coated surface for 30, 60, or 90 minutes at 37°C. Spreading was assessed as the percentage of surface covered and FAK phosphorylation by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (B) CHO-αIIbβ3 cells were pretreated with active MMP-2 (0.75 nM) or pro-MMP-2, heat denatured (Den) MMP-2, MMP-2 treated with TIMP-2 (47.5 nM), TIMP-2 alone, or vehicle for 5 minutes and then layered on a fibrinogen-coated surface for 60 minutes at 37°C. Spreading was assessed as the percentage of surface covered. Data represent means ± SEM (n = 4); *P < .05 vs vehicle. (C) CHO-αIIbβ3 cells were pretreated with active MMP-2 (0.75 nM) or the PEX domain or the catalytic (CAT) domain of MMP-2 (0.75 nM) for 5 minutes and then layered on a fibrinogen-coated surface for 60 minutes at 37°C. Spreading was assessed as the percentage of surface covered. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (D) Gel-filtered human platelets were pretreated with active MMP-2 (0.75 nM) (blue columns) or vehicle (red columns) for 5 minutes and then layered on a fibrinogen-coated surface for 15, 30, or 45 minutes at room temperature. Spreading was assessed as the percentage of surface covered and FAK phosphorylation by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (E) Gel-filtered human platelets were pretreated with active MMP-2 (0.75 nM) for 5 minutes, in the absence or presence of RWJ-56610 (1uM), and then layered on a fibrinogen-coated surface for 30 minutes at room temperature. Results are reported as percent increase in spreading compared with MMP-2-untreated platelets. Data represent means ± SEM (n = 4); *P < .05 vs vehicle.
Effect of MMP-2 on the spreading and FAK phosphorylation of αIIbβ3-expressing CHO cells and platelets. (A) CHO-αIIbβ3 cells were pretreated with active MMP-2 (0.75 nM) (blue columns) or vehicle (red columns) for 5 minutes and then layered on a fibrinogen-coated surface for 30, 60, or 90 minutes at 37°C. Spreading was assessed as the percentage of surface covered and FAK phosphorylation by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (B) CHO-αIIbβ3 cells were pretreated with active MMP-2 (0.75 nM) or pro-MMP-2, heat denatured (Den) MMP-2, MMP-2 treated with TIMP-2 (47.5 nM), TIMP-2 alone, or vehicle for 5 minutes and then layered on a fibrinogen-coated surface for 60 minutes at 37°C. Spreading was assessed as the percentage of surface covered. Data represent means ± SEM (n = 4); *P < .05 vs vehicle. (C) CHO-αIIbβ3 cells were pretreated with active MMP-2 (0.75 nM) or the PEX domain or the catalytic (CAT) domain of MMP-2 (0.75 nM) for 5 minutes and then layered on a fibrinogen-coated surface for 60 minutes at 37°C. Spreading was assessed as the percentage of surface covered. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (D) Gel-filtered human platelets were pretreated with active MMP-2 (0.75 nM) (blue columns) or vehicle (red columns) for 5 minutes and then layered on a fibrinogen-coated surface for 15, 30, or 45 minutes at room temperature. Spreading was assessed as the percentage of surface covered and FAK phosphorylation by western blotting, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (E) Gel-filtered human platelets were pretreated with active MMP-2 (0.75 nM) for 5 minutes, in the absence or presence of RWJ-56610 (1uM), and then layered on a fibrinogen-coated surface for 30 minutes at room temperature. Results are reported as percent increase in spreading compared with MMP-2-untreated platelets. Data represent means ± SEM (n = 4); *P < .05 vs vehicle.
Therefore, to explore the role of different MMP-2 domains, CHO cells expressing αIIbβ3 were incubated with either recombinant MMP-2 catalytic domain (residues 110-466) or MMP-2 PEX domain (residues 467-660). The MMP-2 catalytic domain, while retaining gelatinolytic activity (supplemental Figure 4), did not inhibit CHO cell spreading. On the contrary, the MMP-2 PEX domain, devoid of catalytic activity, reduced spreading as much as full-length MMP-2 (Figure 3C). Therefore, the interaction of MMP-2 with integrin αIIbβ3 depends on the protein’s C terminus.
Interestingly, contrary to what observed with CHO cells, active MMP-2 induced a significant increase of platelet spreading and FAK phosphorylation on fibrinogen (Figure 3D), an effect inhibited by the PAR1 blocker RWJ-56610 (Figure 3E).
The relevance of αIIbβ3 for the activity of MMP-2 on platelets was confirmed by the lack of potentiation of ADP-induced platelet P-selectin expression (Figure 4A) and by the impaired cleavage of PAR1 extracellular domain (Figure 4B) of platelets from Glanzmann thrombasthenia (GT) patients. However, preincubation with abciximab, an αIIbβ3-antagonist monoclonal antibody directed against the RGD-binding pocket and the secondary KQAGDV (lysine-glutamine-alanine-glycine-aspartate-valine)-binding site, or with tirofiban, which recognizes only the RGD-binding sequence, did not affect the priming activity of MMP-2 (supplemental Figure 3D), suggesting that active MMP-2 interacts with integrin αIIbβ3 at a site different from the fibrinogen-binding pocket. Moreover, MMP-2 did not induce αIIbβ3 activation (supplemental Figure 3E), and preactivation of αIIbβ3 by dithiothreitol in CHO cells did not affect PAR1 cleavage by MMP-2 (supplemental Figure 3F).
The effects of active MMP-2 on platelets are abolished in Glanzmann thrombasthenia (GT). (A) Washed platelets from controls or GT patients were preincubated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of ADP (0.5-1 μM). Results are reported as percentage increase CD62P expression vs ADP. Data represent means ± SEM (n = 3); *P < .05 vs control. (B) GT or control washed platelets were treated for 10 minutes with thrombin (1 nM) or MMP-2 (0.75-1.5 nM) at 37°C. PAR1 N terminus was assessed by western blotting with anti–N-terminus PAR1 antibody (Novus Biologicals), and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs unstimulated.
The effects of active MMP-2 on platelets are abolished in Glanzmann thrombasthenia (GT). (A) Washed platelets from controls or GT patients were preincubated with active MMP-2 (0.75 nM) for 5 minutes and then activated with a subthreshold dose of ADP (0.5-1 μM). Results are reported as percentage increase CD62P expression vs ADP. Data represent means ± SEM (n = 3); *P < .05 vs control. (B) GT or control washed platelets were treated for 10 minutes with thrombin (1 nM) or MMP-2 (0.75-1.5 nM) at 37°C. PAR1 N terminus was assessed by western blotting with anti–N-terminus PAR1 antibody (Novus Biologicals), and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .05 vs unstimulated.
PAR1 cleavage by MMP-2 triggers biased signaling
Upon PAR1 activation, the receptor undergoes conformational changes that promote the interaction with G proteins. Thrombin-cleaved PAR1 activates G12/13, Gq, and Gi, triggering a host of intracellular signaling pathways.43
We tested the ability of DPR-TRAP to stimulate the 3 known G-protein-coupled pathways. DPR-TRAP triggered the phosphorylation of p38 MAPK (Figure 5A), showing MAPK activation, and the phosphorylation of Akt (protein kinase B) (Figure 5B), showing PI3K activation, confirming Gq-dependent signaling. DPR-TRAP, as well as MMP-2, elicited Gq-triggered rise in intraplatelet calcium levels, an effect inhibited by the PAR1 antagonist RWJ-56110 (Figure 5C-D). In particular, our results show that DPR-TRAP increases cytoplasmic calcium levels in platelets slightly but significantly (baseline, 95.2 ± 7.9 nmol/L Ca+2; DPR-TRAP, 169.9 ± 16.3 nmol/L Ca+2; n = 3, *P < .05), with kinetics of calcium fluxes different from those triggered by TRAP-6. Indeed, TRAP-6 mediated a rapid and transient increase of Ca+2, whereas DPR-TRAP mediated a delayed and sustained response (supplemental Figure 5A-B). This prolonged signal may be important for the late phases of platelet aggregation and for the priming phenomenon.
Signaling triggered by MMP-2 and DPR-TRAP in human platelets. (A) Platelets were incubated with MMP-2 (0.75 nM), TRAP (10 µM), DPR-TRAP (500 µM), or vehicle (veh) for 5 minutes at 37°C, without (red columns) or with (blue columns) RWJ-56610 (1 µM). Phospho-p38 MAPK was assessed by western blotting, and bands were analyzed using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .005 vs without RWJ-56610. (B) Platelets were incubated with MMP-2 (0.75 nM), TRAP (10 µM), DPR-TRAP (500 µM), or vehicle (veh) for 5 minutes at 37°C, without (red columns) or with (blue columns) RWJ-56610 (1 µM). Phospho-Akt was assessed by western blotting, and bands were analyzed using QUANTISCAN software. Data represent means ± SEM (n = 4); * P < .005 vs without RWJ-56610. (C) Changes in cytosolic free Ca2+ in 5 mM fluo-3–labeled platelets activated with 0.75 nM MMP-2 in the absence or presence of 1 µM RWJ-56610. The agonists were added, and changes in green fluorescence over time were measured in 5 mM fluo-3–loaded GFPs. Data represent means ± SEM (n = 4). (D) Changes in cytosolic free Ca2+ in 5 mM fluo-3–labeled platelets activated with DPR-TRAP (10 and 500 µM) in the absence or presence of RWJ-56610 (1 µM). Agonists were added and changes in green fluorescence in function of time were measured. Data represent means ± SEM (n = 4). (E) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) (red columns) or DPR-TRAP (500 µM) (blue columns) for 5 minutes and then activated with a subthreshold dose of convulxin (1.8 ng/mL) in the presence or absence of U73122 (10 µM). Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 5); *P < .05 vs vehicle. (F) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) (red columns) or DPR-TRAP (500 µM) (blue columns) for 5 minutes and then activated with a subthreshold dose of convulxin (1.8 ng/mL) in the presence or absence of GSK429286 (100 µM). Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 4); *P < .05 vs vehicle. (G) Gel-filtered platelets were preincubated with iloprost (100 ng/mL) for 2 minutes before addition of vehicle (veh), MMP-2 (0.75 nM), TRAP-6 (10 µM), DPR-TRAP (10 and 500 µM), and TRAP-6 (10 µM) plus DPR-TRAP (500 µM). The reaction was stopped after 5 minutes by centrifugation at 12 000g for 2 minutes, pellets were lysed by 0.1 N HCl, and the supernatant was analyzed for cAMP using a competition-based assay. Data represent means ± SEM (n = 4); *P < .05 vs vehicle. (H) Gel-filtered platelets were pretreated with active MMP-2 (0.75 nM) (red columns) or DPR-TRAP (500 µM) (blue columns) for 5 minutes and then activated with a subthreshold dose of convulxin (1.8 ng/mL) without or with 1 µg/mL PTX. Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (I) Gel-filtered platelets were pretreated with active MMP-2 (0.75 nM) without (red columns) or with (blue columns) PTX (1 µg/mL) and then induced to adhere on a fibrinogen-coated surface for 30 minutes. Results are reported as percent increase of platelet spreading. Data represent means ± SEM (n = 3).
Signaling triggered by MMP-2 and DPR-TRAP in human platelets. (A) Platelets were incubated with MMP-2 (0.75 nM), TRAP (10 µM), DPR-TRAP (500 µM), or vehicle (veh) for 5 minutes at 37°C, without (red columns) or with (blue columns) RWJ-56610 (1 µM). Phospho-p38 MAPK was assessed by western blotting, and bands were analyzed using QUANTISCAN software. Data represent means ± SEM (n = 3); *P < .005 vs without RWJ-56610. (B) Platelets were incubated with MMP-2 (0.75 nM), TRAP (10 µM), DPR-TRAP (500 µM), or vehicle (veh) for 5 minutes at 37°C, without (red columns) or with (blue columns) RWJ-56610 (1 µM). Phospho-Akt was assessed by western blotting, and bands were analyzed using QUANTISCAN software. Data represent means ± SEM (n = 4); * P < .005 vs without RWJ-56610. (C) Changes in cytosolic free Ca2+ in 5 mM fluo-3–labeled platelets activated with 0.75 nM MMP-2 in the absence or presence of 1 µM RWJ-56610. The agonists were added, and changes in green fluorescence over time were measured in 5 mM fluo-3–loaded GFPs. Data represent means ± SEM (n = 4). (D) Changes in cytosolic free Ca2+ in 5 mM fluo-3–labeled platelets activated with DPR-TRAP (10 and 500 µM) in the absence or presence of RWJ-56610 (1 µM). Agonists were added and changes in green fluorescence in function of time were measured. Data represent means ± SEM (n = 4). (E) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) (red columns) or DPR-TRAP (500 µM) (blue columns) for 5 minutes and then activated with a subthreshold dose of convulxin (1.8 ng/mL) in the presence or absence of U73122 (10 µM). Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 5); *P < .05 vs vehicle. (F) Gel-filtered platelets were preincubated with active MMP-2 (0.75 nM) (red columns) or DPR-TRAP (500 µM) (blue columns) for 5 minutes and then activated with a subthreshold dose of convulxin (1.8 ng/mL) in the presence or absence of GSK429286 (100 µM). Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 4); *P < .05 vs vehicle. (G) Gel-filtered platelets were preincubated with iloprost (100 ng/mL) for 2 minutes before addition of vehicle (veh), MMP-2 (0.75 nM), TRAP-6 (10 µM), DPR-TRAP (10 and 500 µM), and TRAP-6 (10 µM) plus DPR-TRAP (500 µM). The reaction was stopped after 5 minutes by centrifugation at 12 000g for 2 minutes, pellets were lysed by 0.1 N HCl, and the supernatant was analyzed for cAMP using a competition-based assay. Data represent means ± SEM (n = 4); *P < .05 vs vehicle. (H) Gel-filtered platelets were pretreated with active MMP-2 (0.75 nM) (red columns) or DPR-TRAP (500 µM) (blue columns) for 5 minutes and then activated with a subthreshold dose of convulxin (1.8 ng/mL) without or with 1 µg/mL PTX. Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 3); *P < .05 vs vehicle. (I) Gel-filtered platelets were pretreated with active MMP-2 (0.75 nM) without (red columns) or with (blue columns) PTX (1 µg/mL) and then induced to adhere on a fibrinogen-coated surface for 30 minutes. Results are reported as percent increase of platelet spreading. Data represent means ± SEM (n = 3).
The involvement of Gq-dependent signaling in the activity of MMP-2 on platelets was confirmed by the significant inhibition of the potentiating activity on platelets of MMP-2 and DPR-TRAP by U73122, a phospholipase C inhibitor (Figure 5E).
The involvement of G12/13-dependent signaling was assessed with GSK429286, a selective Rho-kinase inhibitor. Preincubation with GSK429286 markedly reduced the priming effect of MMP-2 and DPR-TRAP on platelets (Figure 5F).
Finally, in order to evaluate whether the effects of MMP-2 on platelets involve Gi-dependent signaling, cAMP was measured. The iloprost-induced increase of intraplatelet cAMP was not affected by DPR-TRAP or MMP-2, in the presence of either the P2Y12 antagonist AR-C66096 or apyrase (supplemental Figure 6A), while it was blunted by TRAP-6 (Figure 5G). Similarly, DPR-TRAP did not affect cAMP levels in resting platelets, while TRAP-6 reduced them (supplemental Figure 6B). Pertussis toxin (PTX), an inhibitor of Gi, did not modify the potentiating activity of MMP-2 or of DPR-TRAP either on platelet activation (Figure 5H) or on platelet spreading (Figure 5I), showing that Gi-dependent signaling is not involved in the activity of MMP-2 on platelets. Moreover, preincubation with the P2Y12 antagonist AR-C66096 did not affect the priming effect of MMP-2 or of DPR-TRAP on platelets (data not shown).
Furthermore, MMP-2 and DPR-TRAP alone did not induce secretion of either ATP or serotonin from dense granules (supplemental Figure 6C,E-F) or of P-selectin from α-granules, differently from TRAP6 (supplemental Figure 6D), and preincubation with MMP-2 before stimulation with serotonin (10-100 μM), a selective Gq activator,44 did not enhance platelet activation.
MMP-2 primes mouse platelets
Human recombinant active MMP-2 potentiates mouse platelet aggregation.10 Given that mouse platelets do not express PAR1 but do express its homolog, PAR3, which does not trigger transmembrane signaling but functions as a cofactor for the activation of PAR4,45 we assessed whether PAR3 is a possible target of MMP-2 by testing the ability of the corresponding aligned MMP-2-generated mouse PAR3 nonapeptide 35TIKSFNGGP (Figure 6A) to potentiate mouse platelet activation and of the PAR4 antagonist tcY-NH2 trans-Cinnamoyl-Tyr-Pro-Gly-Lys-Phe-NH2 (tcY-NH2) to block it. TIKSFNGGP dose-dependently enhanced convulxin-induced mouse platelet P-selectin expression, as well as human active MMP-2 (Figure 6B). The potentiating activity of MMP-2 was strikingly with reduced, although not abolished, platelets from PAR3−/− mice (Figure 6C). On the contrary, TIKSFNGGP potentiated PAR3−/− mouse platelet activation, an effect abolished by the PAR4 inhibitor tcY-NH2 (Figure 6D-E).
Human active MMP-2 and mouse PAR3 TIKSFNGGP peptide potentiate mouse platelet activation. (A) Alignment of human PAR1 and mouse PAR3 exodomains (h, human; m, mouse). (B) Washed mouse platelets were preincubated with active MMP-2 (1.5 nM) or TIKSFNGGP peptide (100-500 µM) for 5 minutes and then activated with a subthreshold dose of convulxin (10 ng/mL). Results are reported as percent increase CD62P expression vs convulxin. Data represent means ± SEM (n = 5). (C) Washed platelets from wild-type (WT) and PAR3−/− mice were pretreated with active MMP-2 (1.5 nM) for 5 minutes and then activated with a subthreshold dose of convulxin (10 ng/mL), and aggregation was followed for 5 minutes. Results are reported as percent increase light transmission aggregometry vs convulxin. Data represent means ± SEM (n = 10); *P < .05 vs WT. (D) Washed platelets from wild-type (WT) (red columns) and PAR3−/− (blue columns) mice were pretreated with active human MMP-2 (1.5 nM) for 5 minutes, without or with tcY-NH2 (1 mM), and then activated with subthreshold dose of convulxin (10 ng/mL). Results are reported as percent increase CD62P expression vs convulxin. Data represent means ± SEM (n = 6); *P < .05 vs WT without tcY-NH2. (E) Washed platelets from WT (red columns) and PAR3−/− (blue columns) mice were pretreated with TIKSFNGGP (500 µM) for 5 minutes, without or with tcY-NH2 (1 mM), and then activated with a subthreshold dose of convulxin (10 ng/mL). Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 4); *P < .05 vs WT without tcY-NH2; #P < .05 vs PAR3−/− without tcY-NH2. (F) Washed platelets from wild-type (red columns) and PAR3−/− (blue columns) mice were pretreated with human active MMP-2 (1.5 nM) for 5 minutes at 37°C, and platelets were lysed. Rho-GTP and total Rho were assessed by western blot analysis, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 4); *P < .05 vs WT baseline; #P < .05 vs WT MMP-2.
Human active MMP-2 and mouse PAR3 TIKSFNGGP peptide potentiate mouse platelet activation. (A) Alignment of human PAR1 and mouse PAR3 exodomains (h, human; m, mouse). (B) Washed mouse platelets were preincubated with active MMP-2 (1.5 nM) or TIKSFNGGP peptide (100-500 µM) for 5 minutes and then activated with a subthreshold dose of convulxin (10 ng/mL). Results are reported as percent increase CD62P expression vs convulxin. Data represent means ± SEM (n = 5). (C) Washed platelets from wild-type (WT) and PAR3−/− mice were pretreated with active MMP-2 (1.5 nM) for 5 minutes and then activated with a subthreshold dose of convulxin (10 ng/mL), and aggregation was followed for 5 minutes. Results are reported as percent increase light transmission aggregometry vs convulxin. Data represent means ± SEM (n = 10); *P < .05 vs WT. (D) Washed platelets from wild-type (WT) (red columns) and PAR3−/− (blue columns) mice were pretreated with active human MMP-2 (1.5 nM) for 5 minutes, without or with tcY-NH2 (1 mM), and then activated with subthreshold dose of convulxin (10 ng/mL). Results are reported as percent increase CD62P expression vs convulxin. Data represent means ± SEM (n = 6); *P < .05 vs WT without tcY-NH2. (E) Washed platelets from WT (red columns) and PAR3−/− (blue columns) mice were pretreated with TIKSFNGGP (500 µM) for 5 minutes, without or with tcY-NH2 (1 mM), and then activated with a subthreshold dose of convulxin (10 ng/mL). Results are reported as percent increase in CD62P expression vs convulxin. Data represent means ± SEM (n = 4); *P < .05 vs WT without tcY-NH2; #P < .05 vs PAR3−/− without tcY-NH2. (F) Washed platelets from wild-type (red columns) and PAR3−/− (blue columns) mice were pretreated with human active MMP-2 (1.5 nM) for 5 minutes at 37°C, and platelets were lysed. Rho-GTP and total Rho were assessed by western blot analysis, and bands were measured using QUANTISCAN software. Data represent means ± SEM (n = 4); *P < .05 vs WT baseline; #P < .05 vs WT MMP-2.
Given that Gq-mediated Ca2+ mobilization and PKC activation are known to be enhanced in PAR3−/− mouse platelets while G12/13- and Gi-mediated signaling are not affected,46 we studied RhoA-GTP activation and found that MMP-2 was not able to increase it in platelets from PAR3−/− mice (Figure 6F). These results confirm the idea that the weak potentiation of the aggregation response by MMP-2 still present in PAR3−/− platelets was due not to a residual MMP-2 stimulatory effect but to the increased Gq signaling pathway of PAR3−/− platelets.
Discussion
Our results unravel a novel mechanism regulating platelet activation that involves the binding of MMP-2 to integrin αIIbβ3 and the subsequent cleavage of PAR1 at a distinct extracellular site triggering biased downstream signaling.
Although unable to act as a full platelet agonist, MMP-2 plays an important role in platelet aggregation by amplifying the activation response to a range of stimuli9 and participates in thrombus formation in vitro47,48 and in vivo,10 but the mechanisms through which MMP-2 facilitates platelet activation have so far remained elusive. We previously showed that the amount of active MMP-2 released in vivo in the coronary circulation of patients with acute coronary syndrome ∼0.625 nM12 and thus is in the range of that reduces found to potentiate platelet activation in vitro. Moreover, in vivo studies in human whole blood showed that the amount of active MMP-2 released at a site of vascular injury is ∼0.270 nM.11 These concentrations may be significantly higher in the microenvironment of a growing thrombus. In fact, it was reported that the thrombus core, compared with the shell, provides an environment for retaining soluble agonists affecting platelet activation, such as thrombin, establishing agonist-specific concentration gradients radiating from the site of damage. Therefore, we can assume that MMP-2 concentrations at sites of vascular injury increase up to the range able to potentiate platelet activation.
We show here that active MMP-2 enhances platelet activation by enzymatically cleaving PAR1 at a specific, noncanonical extracellular site via an αIIbβ3-facilitated mechanism. The cleavage of PAR1 by MMP-2 generates a tethered ligand different from that produced by thrombin that in turn triggers biased PAR1 signaling, initiating Gq- and G12/13-activated, but not Gi-activated, intracellular pathways, thus predisposing platelets to full activation by a subsequent subthreshold stimulus.
We observed that MMP-2 was unable to potentiate aggregation of TRAP-6-desensitized platelets, suggesting a crucial role of PAR1. We then showed that active, but not pro- or inactivated, MMP-2 reduced the expression of the extracellular PAR1 N-terminal domain on platelets, an index of PAR1 cleavage. We then tested the effect of a monoclonal antibody (SPAN12) blocking the N terminus of PAR1, showing that it abolished the potentiating activity of MMP-2 on platelet activation. Therefore we carried out targeted mutation of PAR1 showing that active MMP-2 cleaves the N-terminal extracellular domain of PAR1 between residues 38 and 39 (TL38 ↓D39PR).
A soluble synthetic nonapeptide reproducing the MMP-2-generated tethered ligand (DPR-TRAP) mimicked the effects of MMP-2 on platelets, potentiating agonist-induced platelet P-selectin expression and aggregation but not inducing aggregation directly. Moreover, DPR-TRAP caused PAR1 internalization, a phenomenon occurring after thrombin receptor activation.49
DPR-TRAP stimulated G12/13 and Gq activation in human platelets, as shown by p38-MAPK phosphorylation, intraplatelet Ca+2 increase, and PI3K activation and by the inhibition of MMP-2-priming activity by a Rho-kinase inhibitor and a phospholipase C inhibitor, but not Gi signaling. In fact, MMP-2 and DPR-TRAP did not reduce cAMP levels in iloprost-exposed or resting platelets, although TRAP-6 did.50 Moreover, PTX, which inhibits Gi-receptor coupling, did not affect the potentiating activity of MMP-2 on platelet aggregation. These data are in agreement with our previous results showing that the ADP-scavenger apyrase does not modify the priming effect of MMP-2 on platelets.9
Thus, MMP-2 induces MAPK phosphorylation, PI3K activation, and Ca+2 movement in platelets, but in order to generate full activation, it requires concomitant Gi signaling triggered by other agonists, leading to adenylyl cyclase inhibition and full platelet aggregation. A similar mechanism has been reported for other agents potentiating platelet activation, such as macrophage-derived chemokine51 or PGE2,52 and explains the difference between platelet primers and full platelet agonists.53
Some protease-revealed “noncanonical” tethered-ligand PAR sequences, as well as their soluble peptide agonists, trigger “biased” PAR signaling.54-56 This may be due to the binding of the tethered ligand to specific sites within the receptor pocket, leading to the activation of only some G proteins, or alternatively to a selective diffusion of the activated receptor in the plane of the membrane that allow for interaction with only some effectors.57 The capacity of different agonists to initiate different signaling pathways interacting with the same receptor has been described for many GPCRs, including PAR1. This phenomenon is not surprising, because PAR1 is a flexible protein that interacts with multiple ligands and regulatory proteins that may influence the capacity to signal to particular pathways. It was previously reported that MMP-1 cleaves the PAR1 exodomain at LD39 ↓P40RSFL and that the MMP-1-cleaved receptor or soluble peptide analog (PRSFLLRN) stimulates only G12/13-Rho–dependent pathways,23 unlike MMP-2, which initiates G12/13 and Gq pathways. It is conceivable that the MMP-1-generated peptide PRSFLLRN and the MMP-2-generated peptide DPRSFLLRN bind differently to the PAR1 receptor pocket and/or stabilize diverse conformations of the receptor, leading to the activation of distinct signaling cascades. Mutational studies suggest that different classes of G proteins associate with distinct regions on the cytoplasmic face of PAR1. Then, PRSFLLRN and DPRSFLLRN could bind at or near the G-protein-binding pocket or interact with discrepant residues within the i2 loop, leading to differentiated Gq, Gi, or G12/13 activation.
Secreted MMP-2 localizes to the platelet surface during the early stages of platelet activation,6,11,48 interacting with αIIbβ3.16 Indeed, MMP-2 binds to integrins in other cells, such as to αvβ3 on the surface of angiogenic blood vessels and melanoma cells.58
In order to explore the interaction between MMP-2 and αIIbβ3, we studied the spreading on fibrinogen and FAK phosphorylation of CHO cells expressing integrin αIIbβ3, a simplified system in which talin-triggered signal transduction and other receptors present in platelets are absent. Both active MMP-2 and enzymatically inactive MMP-2 inhibited CHO-cell spreading and FAK phosphorylation, showing that MMP-2 interacts with integrin αIIbβ3 in a noncatalytic, allosteric way and that MMP-2 interferes with the interaction of αIIbβ3 with fibrinogen. However, the MMP-2-αIIbβ3 interaction is not responsible for the platelet-potentiating effect because it is not dependent on the catalytic activity of the enzyme, which is instead strictly required for its priming activity on platelets.7,9,10 Moreover, contrary to what observed with CHO cells, active MMP-2 induced a significant increase of platelet spreading and FAK phosphorylation on fibrinogen, suggesting that the enzyme exerts its effect on platelets by interacting with a surface target different from αIIbβ3. Indeed, preincubation with the PAR1 antagonist RWJ-56610 inhibited the potentiating effect of MMP-2 on platelet spreading.
MMP-2 does not contain a RGD sequence and thus it is not clear how it interacts with αIIbβ3. Using truncated forms of MMP-2, we showed that while the catalytic domain does not block spreading of CHO cells on fibrinogen the PEX domain does, consistent with the established role of the MMP-2 C terminus in the localization to cell surfaces.58 Indeed, a colocalization of αIIbβ3 and αvβ3 with the C terminus of MMP-2 was previously shown with fibrosarcoma and lung cancer cells.59,60
In order to interact with αIIbβ3 on platelets, MMP-2 must be in its active conformation, and this is consistent with previous studies showing that upon activation, the hemopexin-like domain undergoes remodeling, becoming more accessible to ligands.42,61
To further clarify the role of αIIbβ3 in the priming activity of MMP-2 on human platelets, we used CHO cells expressing only PAR1 or both PAR1 and integrin αIIbβ3 as well as platelets from GT patients. Cleavage of PAR1 by MMP-2 was significantly enhanced in CHO cells expressing both PAR1 and αIIbβ3, while MMP-2 was unable to potentiate the activation of platelets of GT patients, confirming that αIIbβ3 is a crucial cofactor for PAR1 activation by MMP-2.
A similar interaction between a proteolytic enzyme and a glycoprotein on platelets was previously reported, showing that GPIb enhances the cleavage of PAR1 by thrombin.62
It can also be hypothesized that the binding to αIIbβ3 plays a role in PAR1-biased signaling by localizing the MMP-2-αIIbβ3-PAR1 complex in a platelet membrane area more prone to interact with Gq and G12/13 than with Gi, but further studies are required.
Given that human MMP-2 primes mouse platelets10 that express PAR3 instead of PAR1, which acts as a cofactor for the activation of PAR4 that in turn triggers intracellular signaling,45 we synthesized the aligned mouse PAR3 nonapeptide (TIKSFNGGP) corresponding to the human PAR1 tethered ligand, showing that it potentiates the activation of both WT and PAR3−/− platelets, therefore acting directly on mouse PAR4. Moreover, the potentiating effect of MMP-2 on PAR3−/− mouse platelets was strikingly reduced, and its priming effect was inhibited by a PAR4 antagonist. Gq-mediated Ca2+ mobilization and PKC activation are increased in PAR3−/− mouse platelets,46 and this may explain why the MMP-2 priming effect on PAR3−/− platelets was not completely abolished. Therefore, we tested RhoA-GTP activation in PAR3−/− mice and found that MMP-2 did not increase activation compared to WT mice. These results suggest that MMP-2 cleaves mouse platelet PAR3, generating a new N-terminal-tethered ligand activating PAR4, according to a previously reported model of cleavage in trans.45
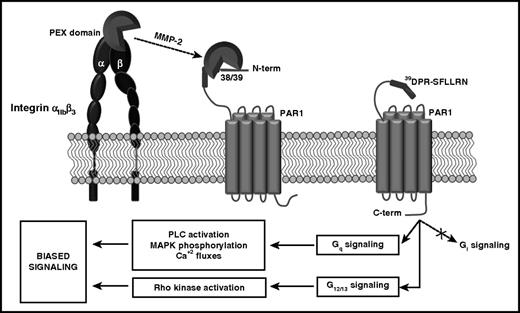
In conclusion, our studies unravel a novel mechanism regulating platelet activation mediated by the αIIbβ3-facilitated cleavage of PAR1 by active MMP-2 at a noncanonical site triggering biased G-protein signaling (Figure 7) and predisposing platelets to full activation by other agonists and identify the MMP-2- αIIbβ3-PAR1 interaction as a potential target for the prevention of arterial thrombosis.
Schematic model of the mechanism of the modulation of platelet signaling by MMP-2. MMP-2, through the PEX domain, interacts with integrin αIIbβ3 on the platelet surface. This interaction facilitates the cleavage of the extracellular domain of PAR1 at the acid aspartic 39 N terminus. MMP-2 cleavage generates an N-terminal (39DPR-TRAP) that interacting with the receptor pocket induces PAR1 biased signaling. In particular, MMP-2 activates the Gq pathway, inducing PAR1-dependent p38-MAPK phosphorylation, phospholipase C (PLC) activation, and Ca+2 fluxes, and the G12/13 pathway, inducing Rho kinase activation, but not the Gi pathway.
Schematic model of the mechanism of the modulation of platelet signaling by MMP-2. MMP-2, through the PEX domain, interacts with integrin αIIbβ3 on the platelet surface. This interaction facilitates the cleavage of the extracellular domain of PAR1 at the acid aspartic 39 N terminus. MMP-2 cleavage generates an N-terminal (39DPR-TRAP) that interacting with the receptor pocket induces PAR1 biased signaling. In particular, MMP-2 activates the Gq pathway, inducing PAR1-dependent p38-MAPK phosphorylation, phospholipase C (PLC) activation, and Ca+2 fluxes, and the G12/13 pathway, inducing Rho kinase activation, but not the Gi pathway.
The demonstration that integrin αIIbβ3 is essential for the cleavage of PAR1 by MMP-2 and the previous observation that MMP-2 is important for arterial thrombosis but much less for primary hemostasis10 may open the way to new strategies for the modulation of platelet responses to thrombogenic stimuli in vivo.
The development of agents targeting the potentiating activity of MMP-2 on platelets may allow for the prevention of thrombosis without increasing bleeding.53
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A.M. Mezzasoma for her help with light transmission aggregometry and cAMP measurements and A. Kuliopulos (Tufts University, Boston, MA) for the kind gift of plasmid pcDEF3.
This work was supported in part by grants from the Telethon Foundation (GGP10155 and GGP15063) (P.G.) and by a fellowship from Fondazione Umberto Veronesi (E.F.).
Authorship
Contribution: P.G. and M.S. designed the study and wrote the manuscript; M.S. performed experiments; S.M. performed experiments involving mouse models; E.F. performed flow cytometry experiments; L.B. participated in the interpretation of cell transfection experiments; and M.F.H. participated in study design and interpretation of data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Gresele, Division of Internal and Cardiovascular Medicine, Department of Medicine, University of Perugia, Via E. dal Pozzo, 06126 Perugia, Italy; e-mail: paolo.gresele@unipg.it.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal