Key Points
Development of treatment resistance through further somatic mutations may occur in Erdheim-Chester disease during BRAF inhibition.
Combinatorial BRAF/MEK inhibition may be beneficial in treatment-resistant ECD harboring a BRAFV600E and further MAPK-activating mutations.
Abstract
Major advances have been made in understanding the pathogenesis of Erdheim-Chester disease (ECD) leading to novel treatment strategies. Targeted therapies such as BRAF inhibition have shown a significant impact on disease management, emphasizing the importance of the activated mitogen-associated protein kinase pathway in this disease. However, incomplete responsiveness, potentially limiting adverse effects, and the occurrence of treatment resistance to BRAF inhibition observed in other BRAF-mutant malignancies imply the importance of therapeutic strategies beyond BRAF inhibition. We report a patient with ECD who carried the BRAFV600E mutation and developed treatment resistance under BRAF inhibition despite initial treatment response. Genetic analyses of a newly developing ECD lesion revealed a somatic KRASQ61H mutation without the presence of BRAFV600E. Accordingly, the addition of MEK-inhibiting trametinib to BRAF-inhibiting dabrafenib was able to overcome acquired partial treatment resistance. This is the first report of treatment resistance as a result of a secondary MAPK pathway–activating mutation during BRAF inhibition in ECD. This case contributes to the ongoing efforts of simultaneous BRAF/MEK inhibition as a promising strategy in ECD.
Introduction
Erdheim-Chester disease (ECD) is a systemic disorder characterized by the pathologic accumulation of non-Langerhans histiocytes.1,2 The identification of a somatic RAS-ERK–activating mutation of the proto-oncogene BRAFV600E in the tumor cells of ECD3 provided the rationale for using BRAF inhibitors such as vemurafenib or dabrafenib.4,5 Yet despite the proposed major role of the RAS-ERK pathway, not all patients harboring the BRAFV600E mutation show complete responsiveness to BRAF inhibition.4-6 Furthermore, severe adverse effects and treatment resistance during BRAF inhibition may occur, as seen in melanoma7 and other BRAF-mutant cancers. Here we report, for the first time, on the clinical efficacy of a combination therapy of the MEK inhibitor trametinib added to dabrafenib in a 54-year-old female patient with disseminated multisystemic ECD who harbored both somatic BRAF and KRAS mutations.
Methods
For metabolic staging of disease activity, fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) was performed on a dedicated whole-body PET/CT scanner (lutetium oxyorthosilicate detectors and 40-row multidetector CT, Siemens Medical Systems, Erlangen, Germany) 60 minutes after intravenous injection of 5 MBq/kg body weight 18F-FDG at blood glucose levels below 10 mmol/L. Images were iteratively reconstructed by using resolution-recovering algorithms (high-definition PET) and interpreted by an experienced nuclear medicine specialist.
Gene sequencing analysis was performed by using the Ion AmpliSeq Cancer Hotspot Panel v2 or the Oncomine Focus Assay with the IonPGM sequencer (all from Thermo Fisher Scientific, San Francisco, CA) according to the manufacturer’s instructions. A run was considered adequate when the average base coverage depth was ≥1000, and the amplicons had at least 500 reads in ≥90%.
Immunohistochemical stainings for CD1a, CD14, CD68, CD163, factor XIIIa, and S100 were performed on an automated immunostainer (Benchmark, Ventana/Roche, Tucson, AZ).
Informed consent to publish this brief report was obtained from the patient.
Results and discussion
A 53-year-old Asian woman was admitted to a regional hospital in April 2007 because of impaired general condition, generalized bone pain, and relapsing fever episodes. Laboratory inflammatory markers (C-reactive protein [CRP], white blood cell count) were increased, and PET/CT scans revealed extensive hypermetabolic bone marrow and multilocular bone involvement in the pelvis, spine, femoral head, and skull, along with a hypermetabolic soft tissue lesion in the right iliac fossa (Figure 1A). Histology of bone marrow and soft tissue biopsies revealed epithelioid foamy histiocytic (CD68+/S100–/CD1a–) infiltration with intermingled giant cells (Figure 2A-B). An integrative clinical, radiologic, and histologic diagnosis of ECD was established. Over the next 5 years, the patient was repeatedly hospitalized because of recurring lower extremity bone pain along with progression of pre-existing and newly developing hypermetabolic lesions in follow-up PET/CT scans. Disease control was not achieved, despite various therapeutic regimens, including prednisone, interferon-α-2a and interleukin-1 blockade with anakinra. The patient was then referred to our hospital.
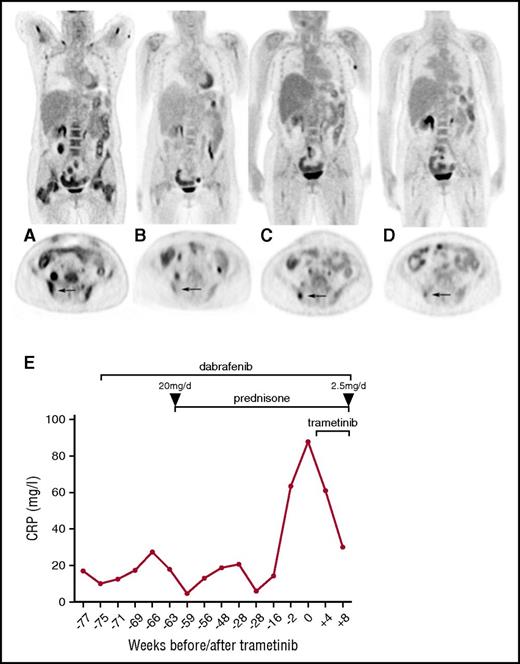
Therapeutic efficacy assessed by FDG-PET/CT scans and serum CRP levels. Coronal and axial FDG-PET/CT images (A) before dabrafenib, (B) best response under dabrafenib, (C) recurrent disease under dabrafenib, and (D) under dual combined dabrafenib/trametinib treatment. Note the bone marrow response under dabrafenib with focal recurrence in the iliac bone (arrow). (E) Longitudinal CRP levels before and after initiation of dabrafenib/trametinib treatment. Prednisone therapy was initiated at 20 mg per day and rapidly tapered to a dose of 2.5 mg per day.
Therapeutic efficacy assessed by FDG-PET/CT scans and serum CRP levels. Coronal and axial FDG-PET/CT images (A) before dabrafenib, (B) best response under dabrafenib, (C) recurrent disease under dabrafenib, and (D) under dual combined dabrafenib/trametinib treatment. Note the bone marrow response under dabrafenib with focal recurrence in the iliac bone (arrow). (E) Longitudinal CRP levels before and after initiation of dabrafenib/trametinib treatment. Prednisone therapy was initiated at 20 mg per day and rapidly tapered to a dose of 2.5 mg per day.
Histologic analysis at initial presentation and clinical relapse. (A-B) Histologic analysis of the bone marrow biopsy at first diagnosis. (A) Foamy histiocytes and scattered giant cells (original magnification ×360; hematoxylin and eosin [H&E] stain,). (B) CD68+ histiocytes (original magnification ×360; immunoperoxidase stain). (C-F) Histologic analysis of the soft tissue biopsy at relapse. (C) Foamy histiocytes and scattered giant cells (original magnification ×200; H&E stain). (D) CD68+ histiocytes (original magnification ×200; immunoperoxidase stain). (F) Factor XIIIa-expressing putative tumor cell equivalents within the histiocytic infiltrate at relapse (original magnification ×200; immunoperoxidase stain). Roughly 25% of the total visible cells express factor XIIIa, which correlates with the detected frequency of KRASQ61H-mutant alleles of 12%. All scale bars represent 50 μm.
Histologic analysis at initial presentation and clinical relapse. (A-B) Histologic analysis of the bone marrow biopsy at first diagnosis. (A) Foamy histiocytes and scattered giant cells (original magnification ×360; hematoxylin and eosin [H&E] stain,). (B) CD68+ histiocytes (original magnification ×360; immunoperoxidase stain). (C-F) Histologic analysis of the soft tissue biopsy at relapse. (C) Foamy histiocytes and scattered giant cells (original magnification ×200; H&E stain). (D) CD68+ histiocytes (original magnification ×200; immunoperoxidase stain). (F) Factor XIIIa-expressing putative tumor cell equivalents within the histiocytic infiltrate at relapse (original magnification ×200; immunoperoxidase stain). Roughly 25% of the total visible cells express factor XIIIa, which correlates with the detected frequency of KRASQ61H-mutant alleles of 12%. All scale bars represent 50 μm.
Given the novel identification of the activating BRAFV600E mutation in the pathogenesis of ECD3 at that time, biopsy samples obtained during the 5 years from initial disease manifestation in 2007 (two soft tissue samples of the nose and the iliac fossa and a bone marrow biopsy) were tested for the presence of that mutation. Indeed, the activating BRAFV600E mutation was identified in all of the biopsies, whereas no KRAS mutation was present. Targeted therapy using dabrafenib (150 mg twice per day) was initiated, and a marked improvement of clinical symptoms, along with a decline of inflammatory markers was achieved. Furthermore, PET/CT re-evaluation revealed reduced metabolic activity in almost all affected regions within 4 weeks of treatment (Figure 1B). CRP levels rose again 4 months after the initiation of dabrafenib without new metabolically active lesions observed on PET/CT scans. Therefore prednisone was added at a maximum dose of 20 mg per day to relieve symptoms of inflammation (fatigue, bone pain) and was rapidly tapered to 2.5 mg per day within 6 months without clinical deterioration or increase of inflammation markers (CRP).
Fourteen months after dabrafenib was initiated and while receiving a stable dose of 2.5 mg per day of prednisone, lower extremity bone pain recurred, and PET/CT scans revealed novel FDG-positive soft tissue lesions in the left upper arm, as well as osseous lesions in the right lower extremity and right iliac bone (Figure 1C). Given that ECD was reactivated despite dabrafenib treatment, we reevaluated the possibility of other activating somatic mutations within the MAPK pathway that had been proposed to drive resistance to BRAF inhibition in other diseases such as metastatic melanoma.7 In a biopsy obtained from the novel PET-positive soft tissue lesion of the upper arm, which was histologically identical to the initial biopsies and which met the criteria for ECD (Figure 2C-F), we detected a previously reported8,9 activating KRAS mutation (KRASQ61H), but the BRAFV600E mutation was not found in this material. Treatment was expanded by the addition of the MEK inhibitor trametinib (2 mg per day), resulting in complete alleviation of symptoms, decreasing CRP levels (Figure 1E), and metabolic response of all former FDG-avid lesions (Figure 1D) assessed 8 weeks later.
Major advances have been made in treating ECD. Former treatment regimens aimed at dampening general inflammatory responses and often caused limiting adverse effects. The identification of the somatic BRAFV600E gain-of-function mutation in Langerhans cell histiocytosis10 and subsequently in ECD3 enabled disease-specific targeted therapy. Indeed, direct BRAF inhibition reportedly led to substantial improvement of disease activity, but it is not equally effective in all patients.4-6 Similarly, our patient initially showed a response after BRAF inhibition but gradually developed resistance to therapy and new ECD lesions. These observations are reminiscent of resistance to BRAF inhibition treatment in BRAFV600E metastatic melanoma that has been associated with mutations alternatively reactivating the MAPK pathway.7 In line with these observations, we identified an alternative MAPK pathway–activating mutation in a clinically BRAF inhibition–resistant lesion and, for the first time, we were able to show that the combination of BRAF and MEK inhibition led to a substantial and rapid improvement in the course of the disease. Interestingly, in contrast to acquired resistance in melanoma,7 the lesion resistant to BRAF-inhibition did not carry the initial BRAF mutation. Furthermore, this is the first description of treatment resistance during BRAF inhibition resulting from a de novo KRASQ61H/BRAFwt mutation occurring in a newly developing lesion. To date, recurrent and MAPK-activating mutations have been identified only in BRAFV600E-wild-type non-Langerhans cell histiocytosis.11 Considering the central importance of the MAPK pathway in systemic histiocytosis, we speculate that various mutations of MAPK pathway components may occur in patients with ECD, ultimately driving histiocyte proliferation through a common pathway. We therefore suggest that mutational analysis of all (anti-BRAF) treatment-resistant ECD lesions should be performed. In addition, combination regimens inhibiting the MAPK-pathway at multiple levels, such as the combination of BRAF and other ERK pathway inhibitors, should be considered in anti-BRAF resistant ECD.
The concept of initiating MEK inhibition in a disease harboring a BRAF mutation is further strengthened by the finding that mutated BRAF is associated with enhanced sensitivity to MEK inhibition.12 Furthermore, we have contributed to the concept of simultaneous BRAF/MEK inhibition as a promising strategy in various pathologic conditions associated with altered MAPK signaling,7,13-15 which is currently under investigation for ECD in a prospective, randomized clinical trial (NCT02281760).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.M.N. organized and analyzed the data, wrote the manuscript, and created Figure 1; F.D.J. organized and analyzed the imaging studies; M.R., C.T.B., and A.W. contributed to the interpretation of the data and reviewed the manuscript; D.K. performed the biopsy procedures and reviewed the manuscript; A.P.-S. analyzed and interpreted the histology and sequencing experiments and reviewed the manuscript; G.C. performed and interpreted the histology and sequencing experiments and reviewed the manuscript; A.T. performed and interpreted the histology and sequencing experiments, wrote part of the manuscript, created Figure 2, and reviewed the manuscript; and T.D. was the primary physician who managed the patient, organized and analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Daikeler, University Hospital Basel, Department of Rheumatology, Petersgraben 4, CH-4031 Basel, Switzerland; e-mail: thomas.daikeler@usb.ch.

![Figure 2. Histologic analysis at initial presentation and clinical relapse. (A-B) Histologic analysis of the bone marrow biopsy at first diagnosis. (A) Foamy histiocytes and scattered giant cells (original magnification ×360; hematoxylin and eosin [H&E] stain,). (B) CD68+ histiocytes (original magnification ×360; immunoperoxidase stain). (C-F) Histologic analysis of the soft tissue biopsy at relapse. (C) Foamy histiocytes and scattered giant cells (original magnification ×200; H&E stain). (D) CD68+ histiocytes (original magnification ×200; immunoperoxidase stain). (F) Factor XIIIa-expressing putative tumor cell equivalents within the histiocytic infiltrate at relapse (original magnification ×200; immunoperoxidase stain). Roughly 25% of the total visible cells express factor XIIIa, which correlates with the detected frequency of KRASQ61H-mutant alleles of 12%. All scale bars represent 50 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/7/10.1182_blood-2016-09-740217/4/m_blood740217f2.jpeg?Expires=1769812268&Signature=rpC9YfhHdMK8AdSgKvlpBQYLgLGWbLT8qOI~zRfAGxmVdyYKW1TgtsfGTJymY2uUqs-5r9y8W7LJc2SQnHpyzKuSFeBt-o3amsYvdtjlXfMnfyW~PalVmRn7qfuszcnaZRpwR~ZQGdY-88vFYJzog4lNZVs~3DfZ5UOxbb~zJWQOyrk7DGeGMicfOrzL2faVAESev04kFlbcUCY3NOvTxhuEEhUVeaVoIEl5jPYXxkRYWpJryJv~0m00QVAANMUlURK7zYR0d3nn50PGFFR4Q6Bjv991KzeZKRntqXdZvq0Ss5jyh1PevBbekZeAqGEPCDRqNojv2~oS5OTRGEj31A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)