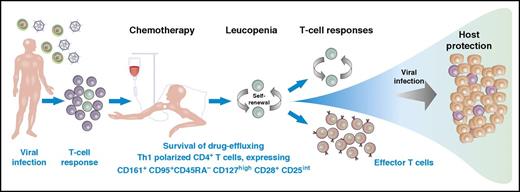
In this issue of Blood, Alsuliman et al describe a CD161+ T-cell population with selectively high toxin effluxing capacity through the MDR1 transporter that helps protect patients with acute myeloid leukemia (AML) during chemotherapy.1
A subpopulation of T cells that express CD161+CD95+CD45RA–CD127hCD28+CD25int are prepared to survive chemotherapy through rapid efflux of toxins and to maintain host protection with a Th1 proinflammatory profile and high expansion potential. This memory T-cell subpopulation is resistant to chemotherapy because of selective expression of the multidrug transporter MDR1. They are quiescent but are capable of self-renewal and proliferation and can differentiate into other cell subsets. These cells express stem-cell–associated markers (c-kit), lack exhaustion markers such as CD57, and display relative quiescence with low Ki-67 expression. This particular subset of memory T cells might play a crucial role for immune reconstitution upon chemotherapy-induced lymphopenia, could be relevant for T-cell memory maintenance under normal conditions, and would be an attractive population for T-cell immunotherapy.
A subpopulation of T cells that express CD161+CD95+CD45RA–CD127hCD28+CD25int are prepared to survive chemotherapy through rapid efflux of toxins and to maintain host protection with a Th1 proinflammatory profile and high expansion potential. This memory T-cell subpopulation is resistant to chemotherapy because of selective expression of the multidrug transporter MDR1. They are quiescent but are capable of self-renewal and proliferation and can differentiate into other cell subsets. These cells express stem-cell–associated markers (c-kit), lack exhaustion markers such as CD57, and display relative quiescence with low Ki-67 expression. This particular subset of memory T cells might play a crucial role for immune reconstitution upon chemotherapy-induced lymphopenia, could be relevant for T-cell memory maintenance under normal conditions, and would be an attractive population for T-cell immunotherapy.
T cells can confer protective immunity toward intracellular target proteins. Different subpopulations of T cells play a key role in distinct immune responses, such as directly killing virally infected or cancerous cells or inducing high-affinity antibody responses in B cells. To maintain protection, long-lived memory T cells are generated, which may persist throughout an individual’s lifespan. Immunotherapy has emerged as a way of treating viral disease and hematologic malignancies given alone or in combination with other types of therapy. Combining chemotherapy with a treatment that relies on functional active T-cell responses requires knowledge of T-cell populations that survive chemotherapy. Interestingly, patients receiving intensive chemotherapy for AML have a low risk of viral complications. Therefore, quiescent T cells with self-renewal capacity must be able to survive this toxic treatment. These quiescent T cells were initially generated after a first infection and may persist for a lifetime.2 The target-dependent T-cell response is controlled by the specificity of the T-cell receptor itself and by the wide heterogeneity and plasticity of CD4+ T helper (Th) cells.3,4
Alsuliman et al studied long-lived pathogen-specific T cells to explore adaptive immune responses in patients undergoing chemotherapy for AML. The authors describe mechanisms underlying long-term persistence of antigen-specific T cells that are related to a subset of memory CD4+ T cells capable of effluxing cellular toxins, including rhodamine (Rho), through the multidrug efflux protein MDR1 (also known as P-glycoprotein and ABCB1). Drug-effluxing CD4+ T cells are characterized as CD161+CD95+CD45RA–CD127hiCD28+CD25int cells (see figure) with a distinct chemokine profile and a Th1-polarized proinflammatory phenotype. For the T-cell response against viral and malignant target structures, effector function is crucial in combination with a strong expansion potential. The required effector functions of CD4+ Th cells are the Th1 cytokines such as interferon-γ, interleukin-2, and tumor necrosis factor. Alsuliman et al describe vigorous proliferation and plasticity of the CD4+CD161+Rho–effluxing T-cell subset. These cells are capable of self-renewal, maintaining their phenotypic and functional characteristics, and giving rise to CD161– progeny. Multidrug effluxing CD4+CD161+ T cells are enriched within the viral-specific Th1 repertoire of healthy donors and AML patients. These cells also survive exposure to daunorubicin chemotherapy in vitro. Multidrug effluxing CD4+CD161+ T cells also resist chemotherapy-induced cytotoxicity in vivo and undergo significant expansion in AML patients who have been rendered lymphopenic after chemotherapy, contributing to the repopulation of antiviral immunity. These findings suggest that CD4+CD161+ T cells with rapid efflux capacity contribute to the maintenance of viral-specific memory T cells during and after chemotherapy. The described characteristics support the hypothesis that this T-cell subset is not only crucial for immune reconstitution during transient lymphopenia but is also a central player in the maintenance of CD4+ T-cell memory in healthy individuals and might be capable of expanding the pool of functionally diverse memory cells.
The identification of a specific long-lived CD4+ T-cell subpopulation that is resistant to chemotherapy and can reconstitute preexisting immunity has important clinical implications, especially for vaccination and adoptive immunotherapy. It might allow monitoring of a sustained immune response after vaccination and could help improve future vaccination strategies. For approaches of adoptive immunotherapy, this subpopulation may be an effective tool for treating viral infections with Th1 cells.5,6 Adoptive T-cell therapy using targeted ex vivo isolation of this unique cell population may be advantageous for sustained therapeutic protection.
The characterization of a quiescent T-cell population that survives chemotherapy and is able to mount functional active T-cell responses also has implications for current immunotherapy approaches against malignancies. Bispecific T-cell engagers recruit endogenous T cells and direct them against leukemia. This treatment relies on the activation and Th1 effector function of those T cells that survive chemotherapy.7,8 The generation of genetically modified T-cell therapies from patient samples during chemotherapy relies on quiescent cells. CD161-expressing memory T cells with superior survival and functional attributes would be ideal candidates. Obviously, this cell population is going to be an exciting and important topic of basic and future clinical research.
Conflict-of-interest disclosure: The author declares no competing financial interests.