In this issue of Blood, Josefsdottir et al provide substantial evidence that commensal gut microbes regulate and sustain normal steady-state hematopoiesis.1
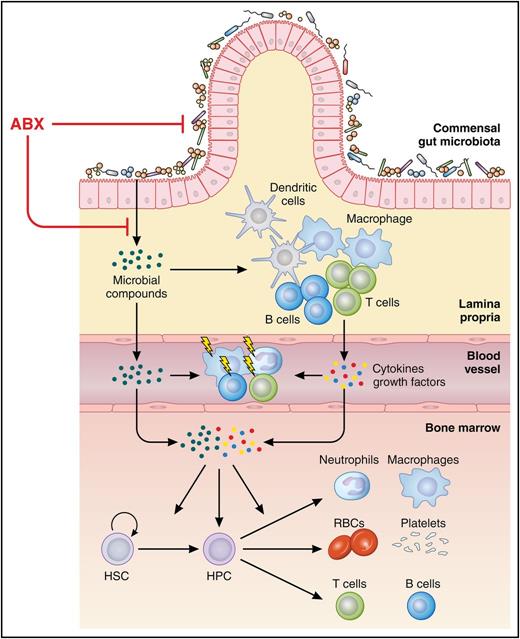
Gut microbiota sustains hematopoiesis. Compounds of the commensal gut microbiota stimulate lymphocytes, macrophages, and dendritic cells in the lamina propria, which, in concert, produce a series of extrinsic stimuli. Together, these microbial and cellular stimuli maintain tonic activity of hematopoietic stem cells (HSC) and hematopoietic progenitor cells (HPC) as well as lymphocytes, monocytes, and neutrophils. Hence, microbial compounds promote steady-state hematopoiesis and vigilance of the innate and adaptive immune system against bacterial and viral infections. Broad-spectrum antibiotic treatment (ABX) can disrupt the balance and diversity of commensal gut microbiota substantially, leading to impaired hematopoiesis and a higher susceptibility to infections. RBC, red blood cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Gut microbiota sustains hematopoiesis. Compounds of the commensal gut microbiota stimulate lymphocytes, macrophages, and dendritic cells in the lamina propria, which, in concert, produce a series of extrinsic stimuli. Together, these microbial and cellular stimuli maintain tonic activity of hematopoietic stem cells (HSC) and hematopoietic progenitor cells (HPC) as well as lymphocytes, monocytes, and neutrophils. Hence, microbial compounds promote steady-state hematopoiesis and vigilance of the innate and adaptive immune system against bacterial and viral infections. Broad-spectrum antibiotic treatment (ABX) can disrupt the balance and diversity of commensal gut microbiota substantially, leading to impaired hematopoiesis and a higher susceptibility to infections. RBC, red blood cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Hematopoiesis is regulated in part by extrinsic regulators, such as growth factors and cytokines, and in part by intrinsic epigenetic and transcriptional regulators that, in concert, orchestrate differentiation of stem cells via a series of progenitor cells into all types of fully mature blood cells.
In their study, Josefsdottir et al demonstrate that broad-spectrum antibiotic treatment of mice for >2 weeks depletes the intestinal microbial flora, which ultimately leads to a decrease in numbers of stem and progenitor cells in the bone marrow and concomitant anemia, leukopenia, and marked pan-lymphopenia. The authors provide substantial experimental evidence that these changes are not a toxic effect of antibiotics on hematopoietic cells, but rather are related to depletion of gut microbiota by antibiotic treatment.
Consistently, the effects of antibiotic treatment were phenocopied in germ-free mice and reversed by fecal microbiota transplantation.1
The molecular mechanisms by which commensal gut microbiota control proper immune function and hematopoiesis were recently shown to partially rely on microbial compounds such as lipopolysaccharides, which sustain steady-state production of neutrophils and their constitutive priming against bacterial infections through Toll-like receptor/MyD88-mediated signaling (see figure).2-4
Importantly, Josefsdottir et al were able to demonstrate that the effects of broad-spectrum antibiotic treatment on hematopoiesis were phenocopied in Stat1 knockout mice, suggesting that microbiota sustain steady-state hematopoiesis through activation of Stat1 signaling. However, further investigations are needed to unravel the type of cells experiencing direct or indirect activation of Stat1 signaling mediated by the commensal gut microbiota.
The novel findings by Josefsdottir et al extend the series of recent studies on host-microbe symbiosis, demonstrating that the gut microbiota is a critical extrinsic regulator of innate and adaptive immunity as well as hematopoiesis, which ultimately maintains the vigilance of the immune system against bacterial and viral infections (see figure).3,5,6 Consistently, perturbation of the balance and diversity in the composition of gut microbiota, referred to as dysbiosis, is associated with higher susceptibility to infections.5,6 Importantly, dysbiosis was also demonstrated to impair clinical response to a variety of cancer treatments including cyclophosphamide, platinum-based therapies, and immunotherapy.7,8 More recently, a single-center study even demonstrated that dysbiosis at the time of engraftment is an independent predictor of mortality after allogeneic stem cell transplantation.9 A follow-up study by the same research group further revealed that treatment of neutropenic fever after allogeneic stem cell transplantation with specific antibiotics (imipenem-cilastatin and piperacillin-tazobactam) leads to dysbiosis and increased graft-versus-host disease–related mortality.10
In the context of these clinical observations, the intriguing data by Josefsdottir et al warrant further studies to investigate whether restoration of commensal gut microbiota in immunocompromised and antibiotic-treated cancer patients by fecal microbiota transplantation may improve clinical outcome after chemotherapy and immunotherapy as well as allogeneic stem cell transplantation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal