Key Points
POAML (specifically Ann Arbor stage I disease) has an excellent clinical outcome, with only a few patients succumbing to lymphoma.
POAML patients face a continuous risk of distant relapse, including in the central nervous system, and transformation to aggressive lymphoma.
Abstract
While primary ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphoma (POAML) is the most common orbital tumor, there are large gaps in knowledge of its natural history. We conducted a retrospective analysis of the largest reported cohort, consisting of 182 patients with POAML, diagnosed or treated at our institution to analyze long-term outcome, response to treatment, and incidence and localization of relapse and transformation. The majority of patients (80%) presented with stage I disease. Overall, 84% of treated patients achieved a complete response after first-line therapy. In patients with stage I disease treated with radiation therapy (RT), doses ≥30.6 Gy were associated with a significantly better complete response rate (P = .04) and progression-free survival (PFS) at 5 and 10 years (P < .0001). Median overall survival and PFS for all patients were 250 months (95% confidence interval [CI], 222 [upper limit not reached]) and 134 months (95% CI, 87-198), respectively. Kaplan-Meier estimates for the PFS at 1, 5, and 10 years were 91.5% (95% CI, 86.1% to 94.9%), 68.5% (95% CI, 60.4% to 75.6%), and 50.9% (95% CI, 40.5% to 61.6%), respectively. In univariate analysis, age >60 years, radiation dose, bilateral ocular involvement at presentation, and advanced stage were significantly correlated with shorter PFS (P = .006, P = .0001, P = .002, and P = .0001, respectively). Multivariate analysis showed that age >60 years (hazard ratio [HR] 2.44) and RT<30.6Gy (HR=4.17) were the only factors correlated with shorter PFS (P = .01 and P = .0003, respectively). We demonstrate that POAMLs harbor a persistent and ongoing risk of relapse, including in the central nervous system, and transformation to aggressive lymphoma (4%), requiring long-term follow-up.
Introduction
Primary ocular adnexal lymphomas (POALs) are rare malignant lymphoid proliferations primarily affecting the orbit, lacrimal gland, conjunctiva, and eyelids. POAL is the most common type of cancer affecting these anatomical sites, accounting for 55% of all orbital tumors.1 POALs comprise ∼1% to 2% of all non-Hodgkin’s lymphomas (NHLs) and 5% to 15% of extranodal lymphomas.2,3 While different subtypes of NHL may present as POAL, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) is the most common histological subtype, accounting for 38% to 64% of POAL cases.4-7
Multiple retrospective studies have described clinical and epidemiological features of primary ocular adnexal MALT lymphoma (POAML), including female preponderance, frequent occurrence in the orbit, and increased incidence of bilateral involvement.8 Association with Chlamydia psittaci infection in certain geographic areas has also been reported.9 The pathogenesis of these tumors was elucidated by an investigation of the prevalence of chromosomal translocations and mutations, which showed alterations in genes that regulate cell survival and apoptosis pathways, as well as through interrogation of immunoglobulin gene usage and analysis of somatic mutations.9-11 These studies implicated a role for autoantigen-induced activation of the B-cell receptor in the pathogenesis of POAML.12 While major advances were achieved, a large gap in knowledge of POAML exists, primarily due to the rarity of this disease. The majority of POAML clinical studies are small and retrospective with limited follow-up, resulting in nonreproducible findings and incomplete understanding of the natural history, incidence of transformation, relapse, and dissemination of this disease. These limitations also explain the conflicting reports regarding the association between various clinical parameters (eg, age, anatomical location, and bilaterality) and disease recurrence and progression-free survival (PFS).13-15 Moreover, the absence of prospective randomized phase 3 studies has resulted in no official level I recommendations on optimal treatment. Radiation therapy (RT) is currently a well-accepted treatment option for limited stage POAML, resulting in up to 85% to 100% long-term complete remission (CR) rates.8,16,17 However, cohort size and follow-up duration are limited in many of these studies. Furthermore, there is no consensus on the optimal radiation dose.
Consequently, we present the largest single-institute study on POAML, consisting of 182 patients followed for up to 387 months, in which we specifically analyzed outcomes, interrogated for an association between lymphoma progression and clinical parameters at presentation, and investigated the role of radiation dose on local and systemic relapse. While not substituting for the need of prospective studies, this study addresses at least some of the gaps in our knowledge and will provide useful guidelines for treating these patients.
Materials and methods
Patients
Between January 1984 and December 2015, 182 patients with POAML were seen at the Sylvester Comprehensive Cancer Center, Bascom Palmer Eye Institute, and Jackson Memorial Hospital at the University of Miami. These patients were identified by review of the University of Miami tumor registry and pathology database. Ninety of these patients were reported previously,8 but their clinical follow-up was updated for this study. The institutional review board of the University of Miami approved this retrospective study, which followed the tenets of the Declaration of Helsinki. To be included in this study, patients were required to have biopsy material that met morphologic and immunophenotypic diagnostic criteria of MALT lymphoma defined by the 2008 World Health Organization classification.18 All pathology specimens were reviewed by expert hematopathologists and showed B-cell monoclonality. Ninety-two specimens were evaluated for the presence of C psittaci, as previously reported,19 and all were negative.
All cases were retrospectively studied to collect complete clinical information on patient demographics, Eastern Cooperative Oncology Group performance status, relevant medical history, presenting symptoms, anatomic location of the lesions, International Prognostic Index and information on each of its components, laboratory data, imaging studies, stage, details of treatment, response to therapy, timing and location of recurrence, management of recurrent or progressive disease, PFS, overall survival (OS), second-line therapy and disease status at last follow-up. The radiation oncology database was reviewed to extract all available information on radiation therapy.
Staging evaluation was not standardized during the study interval but included ophthalmological and complete physical examination, hematological and chemical survey with lactate dehydrogenase (LDH), magnetic resonance imaging, or computed tomography of the orbit, chest, abdomen, and pelvis. The decision to perform staging bone marrow (BM) biopsy and serum protein electrophoresis was at the discretion of the treating oncologist; they were routinely performed in patients seen by the senior author.
Primary ocular adnexa disease sites were defined as involvement of orbit, conjunctiva, lacrimal gland, or eyelid, based on ophthalmic examination and radiological studies. Bilateral ocular adnexal involvement was designated as Ann Arbor stage IE disease, according to the current Ann Arbor staging classification.20 Ann Arbor stage IE tumors were further categorized according to the tumor, node, metastases (TNM)–based American Joint Committee on Cancer (AJCC) International Union Against Cancer ocular lymphoma staging system.21
Treatment
Patients were treated according to the disease stage at the discretion of the treating physician. Patients with localized lymphoma were most commonly treated with RT. RT for orbital tumors that are not conjunctival was usually performed by treating the entire orbit using a wedge pair technique to include dosing to the conus, although some patients, especially more recently, were treated by intensity-modulated RT. In the case of palpebral or bulbar conjunctival lymphomas, we have preferred a mixed photon and high-energy electron beam in order to bring the dosing closer to the surface of the orbit. The radiation dose was initially defined by the treating radiation oncologist and ranged from 22.0 to 45.0 Gy, in general gradually evolving from 36 Gy in 20 fractions downward to 30.6 Gy in 17 fractions (or 30 Gy in 15 fractions) as the targeted dose. After 2003, the 30.6 Gy dose became uniformly standard at our institution.
Statistical analysis
OS was defined as the time from diagnosis (biopsy) to death or last follow-up. PFS was computed from the time of diagnosis until disease progression, transformation, relapse, or death, with censoring of patients who remained disease-free at the time of last follow-up. Time to progression (TTP) was computed from the time of diagnosis until disease progression, transformation, or relapse, with censoring of patients who remained disease-free at the time of last follow-up. Patients with no information on treatment were excluded from OS, PFS, and TTP analyses. Kaplan-Meier survival curves were constructed to compare subgroups using the log-rank test. Demographic and clinical characteristics were analyzed using the Student t test and χ2 test. Multivariate analyses on PFS and OS were performed with a Cox proportional hazards regression model including the variables that were significant in univariate analysis. A P value < .05 was considered statistically significant. JMP version 12 (1989-2007; SAS Institute, Cary, NC) and R version 3.2.3 were used for the statistical analyses.
Results
Patient characteristics and clinical presentation
Between 1984 and 2015, 182 patients with POAML were diagnosed and/or received treatment at our institutions. Table 1 summarizes their demographic and clinical characteristics. Median age at diagnosis was 63 years (range, 7-92 years). The female-to-male ratio was 1.5:1. This was mainly due to a female-to-male ratio of 2:1 in patients with conjunctival presentation, and there was no marked gender difference in patients with POAML located in the orbit and lacrimal gland. Patients with lacrimal gland POAML tended to present at a younger age (median, 49.5 years; range, 21-91 years) compared with those with orbital POAML (median, 65 years; range, 7-90 years) (P = .002) and conjunctival POAML (median, 62 years; range, 7-92 years) (P = .05). The ratio of non-Hispanic patients to Hispanic patients was 2:1, with similar racial distribution at different anatomical locations.
Demographic and selected clinical characteristics of 182 patients with POAML
| Characteristic . | Number . |
|---|---|
| Age >60 y | 100 (54.9%) |
| Median age (range), y | 63 (7-92) |
| Males/females | 72/110 |
| Race | |
| Non-Hispanic | 123 (67.5%) |
| Hispanic | 59 (32.9%) |
| Anatomical location | |
| Conjunctival | 51 (28%) |
| Orbit | 102 (56%) |
| Lacrimal gland | 29 (15.9%) |
| Bilateral disease | 28 (15.6%) |
| Ann Arbor stage/AJCC stage* | |
| I | 145 (79.6%) |
| T1 | 46 (31.7%) |
| T2 | 92 (63.4%) |
| T3 | 5 (3.4%) |
| T4 | 2 (1.3%) |
| II | 5 (2.7%) |
| III | 9 (4.9%) |
| IV | 16 (8.7%) |
| LDH above normal† | 11 (11.2%) |
| BM involvement‡ | 10 (8.6%) |
| IPI score | |
| 0 | 40 (22%) |
| 1 | 41 (22.5%) |
| 2 | 10 (5.4%) |
| 3 | 7 (3.8%) |
| 4 | 1 |
| NA | 83 (45.6%) |
| Characteristic . | Number . |
|---|---|
| Age >60 y | 100 (54.9%) |
| Median age (range), y | 63 (7-92) |
| Males/females | 72/110 |
| Race | |
| Non-Hispanic | 123 (67.5%) |
| Hispanic | 59 (32.9%) |
| Anatomical location | |
| Conjunctival | 51 (28%) |
| Orbit | 102 (56%) |
| Lacrimal gland | 29 (15.9%) |
| Bilateral disease | 28 (15.6%) |
| Ann Arbor stage/AJCC stage* | |
| I | 145 (79.6%) |
| T1 | 46 (31.7%) |
| T2 | 92 (63.4%) |
| T3 | 5 (3.4%) |
| T4 | 2 (1.3%) |
| II | 5 (2.7%) |
| III | 9 (4.9%) |
| IV | 16 (8.7%) |
| LDH above normal† | 11 (11.2%) |
| BM involvement‡ | 10 (8.6%) |
| IPI score | |
| 0 | 40 (22%) |
| 1 | 41 (22.5%) |
| 2 | 10 (5.4%) |
| 3 | 7 (3.8%) |
| 4 | 1 |
| NA | 83 (45.6%) |
IPI, International Prognostic Index; NA, not available.
Seven patients did not have staging information.
Analyzed in 98 patients.
BM was performed in 116 patients. From the 66 patients who did not undergo BM biopsy at presentation, 54 had Ann Arbor stage I disease, 1 had Ann Arbor stage II disease, and 4 had Ann Arbor stage III based on imaging studies.
The majority of patients with available information had an Eastern Cooperative Oncology Group performance status of 0 or 1. Relevant previous history was remarkable for prior diagnosis of autoimmune diseases, cancer, and eye disorders in 18 patients (12%), 26 patients (19%), and 26 patients (19%), respectively (supplemental Table 1, available on the Blood Web site). Chronic hepatitis B and hepatitis C infections were detected in 2 (5.8%) and 5 (14.7%) of 34 tested patients, respectively. Only 1 patient was HIV positive. Fifty-eight patients underwent blood examination for the presence of monoclonal gammopathy, which was detected in 12 patients (20.6%) (age range, 19-92 years), and the types were immunoglobulin M (IgM) in 6, IgG in 4, and IgA in 2 patients.
POAML was located in the orbit, conjunctiva, and lacrimal gland in 102 patients (56%), 51 patients (28%), and 29 patients (16%), respectively. Four patients with orbital PAOML and 1 patient with conjunctival PAOML had concomitant eyelid involvement (stage T3 based on the AJCC-TNM staging). In subsequent analyses, these patients were included in the orbital and conjunctival cohorts, respectively. One patient had concomitant involvement of the orbit and the lacrimal gland (included in the orbital group as per the AJCC-TNM staging, which includes orbital with lacrimal gland involvement in T2b stage).
Ocular symptoms were the major initial reason to seek medical attention in all the patients but varied according to primary tumor sites. Visible lesions (eg, salmon patches) were more common in patients with conjunctival lymphoma, whereas orbital and lacrimal tumors usually presented with periorbital edema, proptosis, or palpable masses. None of the patients had extraorbital (nonophthalmic) complaints or B-symptoms.
At the time of presentation, 145 (80%) patients had Ann Arbor stage I disease, including 46 patients (32%) with a TNM-AJCC stage of T1N0M0, 92 patients (63%) with T2N0M0, 5 patients with T3N0M0, and 2 patients with T4N0M0. Two patients had T2c disease with extraocular muscle involvement. Two patients presented with T4 tumors (pterygopalatine fossa and cavernous sinus involvement, respectively). Twenty-eight patients (16%) had bilateral ocular involvement (11 conjunctival, 4 lacrimal, and 13 orbital), including 21 patients with Ann Arbor stage I, 4 with stage III, and 3 with stage IV disease. Other extraocular sites involved by lymphoma at presentation included the spleen and lung (each in 3 patients) and the skin, kidney, oropharyngeal, central nervous system (CNS) and noncontiguous nasal cavity (in 1 patient each). BM was involved in 10 patients (9%) who underwent BM biopsy (AJCC stage M1b; Table 1). Had a BM biopsy not been performed, 6 of these patients would have been staged as Ann Arbor stage I/II disease. AJCC stage M1a disease was present in 6 patients with a negative BM biopsy result but with involvement of lung (3 patients), CNS, oropharynx, and subcutaneous nodules.
Treatment, response, and follow-up
A total of 174 patients (142 patients with Ann Arbor stage I, 5 patients with Ann Arbor stage II, 9 patients with Ann Arbor stage III, 16 patients with Ann Arbor stage IV, and 2 patients without complete staging) had available data on first-line treatment and response. Table 2 and supplemental Table 2 summarize treatment modalities, response rates, relapses, and their anatomical distribution (local vs systemic).
First-line therapy and CR rates stratified according to disease stage at presentation
| Ann Arbor stage . | First-line therapy . | Number of patients (174)* . | CR . | Relapse or progression type† . | ||
|---|---|---|---|---|---|---|
| Systemic . | Local . | Combined . | ||||
| I | RT only | 111 | 108 | 17 | 2 | 3 |
| RT + other‡ | 12 | 11 | 2 | 1 | 0 | |
| Chemotherapy | 7 | 7 | 1 | 1 | 0 | |
| Other‡ | 12 | 6 | 1 | 3 | 0 | |
| II | RT only | 2 | 2 | 1 | 0 | 0 |
| RT + other | 1 | 1 | 0 | 0 | 0 | |
| Chemotherapy | 1 | 0 | 0 | 0 | 0 | |
| Other | 1 | 1 | 0 | 0 | 0 | |
| III | RT + other | 1 | 0 | 1 | 0 | 0 |
| Chemotherapy only | 3 | 0 | 0 | 2 | 0 | |
| Other | 5 | 1 | 1 | 1 | 0 | |
| IV | RT only | 4 | 0 | 3 | 0 | 0 |
| RT + other | 5 | 5 | 3 | 0 | 1 | |
| Chemotherapy only | 4 | 2 | 0 | 2 | 0 | |
| Other | 3 | 2 | 0 | 2 | 0 | |
| Ann Arbor stage . | First-line therapy . | Number of patients (174)* . | CR . | Relapse or progression type† . | ||
|---|---|---|---|---|---|---|
| Systemic . | Local . | Combined . | ||||
| I | RT only | 111 | 108 | 17 | 2 | 3 |
| RT + other‡ | 12 | 11 | 2 | 1 | 0 | |
| Chemotherapy | 7 | 7 | 1 | 1 | 0 | |
| Other‡ | 12 | 6 | 1 | 3 | 0 | |
| II | RT only | 2 | 2 | 1 | 0 | 0 |
| RT + other | 1 | 1 | 0 | 0 | 0 | |
| Chemotherapy | 1 | 0 | 0 | 0 | 0 | |
| Other | 1 | 1 | 0 | 0 | 0 | |
| III | RT + other | 1 | 0 | 1 | 0 | 0 |
| Chemotherapy only | 3 | 0 | 0 | 2 | 0 | |
| Other | 5 | 1 | 1 | 1 | 0 | |
| IV | RT only | 4 | 0 | 3 | 0 | 0 |
| RT + other | 5 | 5 | 3 | 0 | 1 | |
| Chemotherapy only | 4 | 2 | 0 | 2 | 0 | |
| Other | 3 | 2 | 0 | 2 | 0 | |
Two patients did not have staging and received surgical treatment, with 1 patient achieving CR.
Number of relapses or progression in all patients irrespective of the response to first-line therapy.
For details, see supplemental Table 2.
RT was the primary treatment modality in 117 patients, including 111 patients with Ann Arbor stage I disease. Only 3 (2.7%) of these Ann Arbor stage I disease patients did not achieve CR. Data on the RT dose in patients with Ann Arbor stage I disease were available for 98 patients. The dose of RT varied over the study period, ranging from 22.0 to 45.0 Gy (median, 30.6 Gy). Twenty patients received RT at dose of <30.6 Gy (mean dose, 29 Gy), with 18 achieving CR and 2 achieving partial remission (PR) (they received 22 and 24 Gy doses). Seventy-eight patients received RT at a dose ≥30.6 Gy (mean dose, 33.6 Gy), with all but 1 patient achieving CR and the remaining patient achieving PR (received 36 Gy, had a residual mass that never progressed, and never experienced systemic relapse) (P = .04). With a median follow-up of 63 months (range, 2-387), the incidence of local relapse in the irradiated eye was significantly different (4 with a dose of <30.6 Gy and 1 with a dose ≥30.6 Gy; P = .0058). In addition, in patients achieving initial CR, the incidence of all relapses was also significantly different (P = .007) with different doses of RT. With a RT dose <30.6 Gy, relapses were observed between 2 months and 8 years after radiation in 9 patients (50%) (locally in the irradiated eye in 2 patients and systemic in 7 patients, including 2 patients with ipsilateral and 3 patients with contralateral eye relapses). In contrast, with RT at a dose ≥30.6 Gy, systemic relapses were observed between 7 months and 16.5 years after radiation in 11 patients (14%) (including 1 patient with concomitant relapse in the irradiated eye and 2 patients with concomitant relapse in the contralateral eye). None of the relapses in these patients were exclusively limited to the initially irradiated eye.
Five patients (4.5%) treated with RT for Ann Arbor stage I disease developed recurrent disease in the CNS. Three of these patients received RT at a dose <30.6 Gy, and 2 patients received RT at a dose ≥30.6 Gy (supplemental Table 3). In addition, 2 patients with Ann Arbor stage II disease received involved-field RT to all sites of lymphoma, and RT was also used in 4 patients with Ann Arbor stage IV disease. Two of these patients had disease in the BM in addition to ocular adnexa tumor, and 1 patient presented with extensive local spread to the CNS that could be incorporated in a single radiation field. Side effects of RT are summarized in supplemental Table 4.
Treatment and response in other patients with Ann Arbor stage I and in patients with stage II to IV disease are summarized in Table 2. Overall, initial therapy led to CR in 147 patients (84%) and PR in 14 patients (8%) (3 patients in stage I, 1 patient in stage II, 4 patients in stage III, and 6 patients in stage IV), stable disease in 3 patients (2 patients in stage II and 1 patient in stage IV), and progressive disease in 5 patients (2 patients in stage I not treated initially with RT and 3 patients in stage III).
Survival and outcome
Follow-up data were available for 174 patients with a median follow-up of 63.5 months (range, 1-387 months). During this period, 20 patients died, but death was directly attributed to lymphoma in only 3 of these cases. Transformation to diffuse large B-cell lymphoma was observed in 7 patients (4%), leading to death in 2 patients.
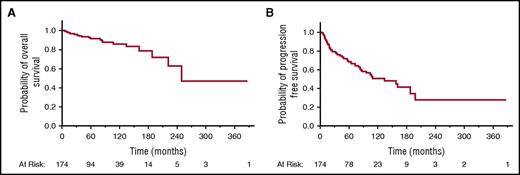
Median OS for all patients was 250 months (95% confidence interval [CI], 222 to upper limit not reached) (Figure 1A). The median lymphoma-specific OS time was not reached. The median PFS for all patients was 134 months (95% CI, 87-198) (Figure 1B). Kaplan-Meier estimates for the PFS at 1, 5, and 10 years were 91.5% (95% CI, 86.1% to 94.9%), 68.5% (95% CI, 60.4% to 75.6%), and 50.9% (95% CI, 40.5% to 61.1%), respectively. The median TTP for all patients was 198 months (95% CI, 109 to upper limit not reached). Kaplan-Meier estimates for the TTP at 1, 5, and 10 years after completion of the first-line treatment were 92.7% (95% CI, 87.6% to 95.8%), 73.8% (95% CI, 65.8% to 80.4%), and 60% (95% CI, 49% to 70.0%), respectively. Of the 147 patients at Ann Arbor stages I to IV who achieved CR after first-line treatment, 5 (3.4%), 24 (16.3%), and 4 (2.7%) eventually developed local, extraorbital, or combined relapse, respectively. Even patients with an initial Ann Arbor stage IE disease exhibited relapses that occurred continuously over time with estimates of cumulative relapse or progression of 5.1%, 17.5%, and 31% at 1, 5, and 10 years, respectively.
Prognostic factors
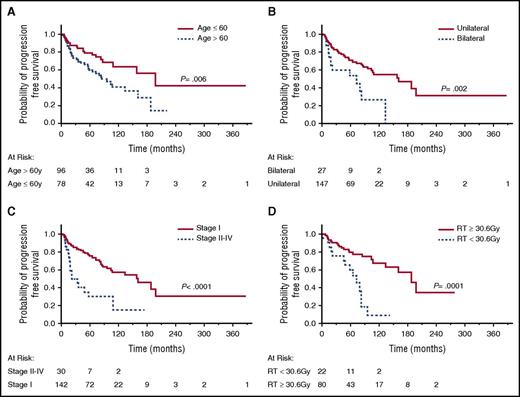
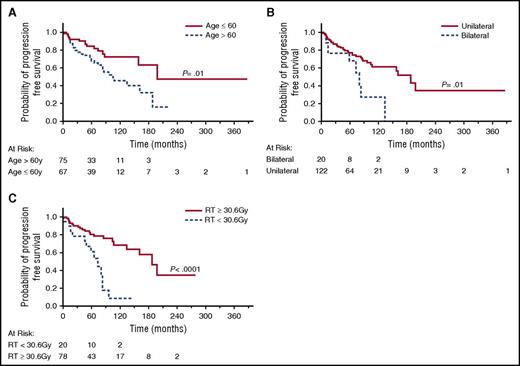
Associations between age, sex, race, stage, LDH levels, location, bilaterality, treatment, dose of RT, and outcome were examined, and identified prognostic factors are shown in Table 3 and Figure 2A-D. None of these factors were associated with lymphoma-specific OS. Patients >60 years had significantly shorter OS (P = .0001) and PFS (P = .006) than younger patients. While the anatomical location of the lymphoma was not associated with OS or PFS, patients with bilateral involvement at presentation had shorter OS (P = .0003) and PFS (P = .002) compared with patients with unilateral disease. Ann Arbor stage II to IV disease was also associated with shorter PFS (P = .0001). There was no difference in OS and PFS between patients who underwent BM biopsy and aspiration and those who did not. Dose of RT at initial therapy (≥30.6 Gy vs <30.6 Gy) was correlated with PFS, but not OS. Similarly, significant differences in PFS were observed also between patients receiving RT at doses of >30 Gy vs ≤30 Gy (P < .0001) and doses of ≥36 Gy vs <36 Gy (P = .0007) (data not shown). In contrast, patients’ race, gender, LDH level, and AJCC stage T1N0M0 vs T2N0M0 were not associated with OS or PFS. Multivariate analysis showed that age >60 years and RT <30.6 Gy were independently associated with shorter PFS (Table 4). In patients with Ann Arbor stage I disease, age >60 years, bilaterality and RT <30.6 Gy were independently associated with shorter PFS in univariate analysis (Figure 3A-C), but in multivariate analysis, only age >60 years and RT <30.6 Gy were significantly associated with shorter PFS.
Univariate analysis of factors associated with PFS in patients with POAML
| Factor . | PFS (mo) . | |
|---|---|---|
| Median (95% CI) . | P . | |
| Age (y) | ||
| ≤60 | 198 (109 to not reached) | .006 |
| >60 | 85 (57-161) | |
| Ann Arbor stage | ||
| I | 161 (104 to not reached) | .0001 |
| II to IV | 22 (19-57) | |
| Laterality | ||
| Bilateral | 73 (19-134) | .002 |
| Unilateral | 161 (104 to not reached) | |
| RT dose | ||
| <30.6 Gy | 73 (46-83) | .0001 |
| ≥30.6 Gy | 188 (134 to not reached) | |
| Factor . | PFS (mo) . | |
|---|---|---|
| Median (95% CI) . | P . | |
| Age (y) | ||
| ≤60 | 198 (109 to not reached) | .006 |
| >60 | 85 (57-161) | |
| Ann Arbor stage | ||
| I | 161 (104 to not reached) | .0001 |
| II to IV | 22 (19-57) | |
| Laterality | ||
| Bilateral | 73 (19-134) | .002 |
| Unilateral | 161 (104 to not reached) | |
| RT dose | ||
| <30.6 Gy | 73 (46-83) | .0001 |
| ≥30.6 Gy | 188 (134 to not reached) | |
PFS of all POAML patients. (A) PFS in patients aged <60 years vs ≥60 years. (B) PFS in patients with bilateral vs unilateral eye disease. (C) PFS in patients with Ann Arbor stage I disease vs stage II to IV disease. (D) PFS in patients treated with RT <30.6 Gy vs ≥30.6 Gy.
PFS of all POAML patients. (A) PFS in patients aged <60 years vs ≥60 years. (B) PFS in patients with bilateral vs unilateral eye disease. (C) PFS in patients with Ann Arbor stage I disease vs stage II to IV disease. (D) PFS in patients treated with RT <30.6 Gy vs ≥30.6 Gy.
Multivariate analysis of factors associated with PFS in all patients with POAML and and Ann Arbor stage I disease
| Factor . | Coefficient . | z score . | PFS (P value) . |
|---|---|---|---|
| Patients with POAML | |||
| Age (>60 y vs ≤60 y) | 0.862 | 2.44 | .015 |
| Eye involvement (bilateral vs unilateral) | 0.108 | 0.22 | NS |
| RT dose (≥30.6 Gy vs <30.6 Gy) | −1.484 | −4.18 | .00003 |
| Stage (I vs II to IV) | 1.05 | 1.60 | NS |
| Patients with Ann Arbor stage I | |||
| Age (>60 y vs ≤60 y) | 1.15 | 3.02 | .0025 |
| Eye involvement (unilateral vs bilateral) | −0.26 | −0.50 | NS |
| RT dose (≥30.6 Gy vs <30.6 Gy) | −1.74 | −4.59 | .000004 |
| Factor . | Coefficient . | z score . | PFS (P value) . |
|---|---|---|---|
| Patients with POAML | |||
| Age (>60 y vs ≤60 y) | 0.862 | 2.44 | .015 |
| Eye involvement (bilateral vs unilateral) | 0.108 | 0.22 | NS |
| RT dose (≥30.6 Gy vs <30.6 Gy) | −1.484 | −4.18 | .00003 |
| Stage (I vs II to IV) | 1.05 | 1.60 | NS |
| Patients with Ann Arbor stage I | |||
| Age (>60 y vs ≤60 y) | 1.15 | 3.02 | .0025 |
| Eye involvement (unilateral vs bilateral) | −0.26 | −0.50 | NS |
| RT dose (≥30.6 Gy vs <30.6 Gy) | −1.74 | −4.59 | .000004 |
NS, not significant.
PFS of stage I POAML patients. (A) PFS in patients aged <60 years vs ≥60 years. (B) PFS in patients with bilateral vs unilateral eye disease. (C) PFS in patients treated with RT <30.6 Gy vs ≥30.6 Gy.
PFS of stage I POAML patients. (A) PFS in patients aged <60 years vs ≥60 years. (B) PFS in patients with bilateral vs unilateral eye disease. (C) PFS in patients treated with RT <30.6 Gy vs ≥30.6 Gy.
Discussion
To our knowledge, this is the largest analysis on the long-term course of patients with POAML. Overall, 182 patients were analyzed, with a median observation time of 63.5 months. The major findings are as follows: Patients with POAML (specifically stage I disease) had an excellent clinical outcome, with only a few succumbing to lymphoma. The median lymphoma-specific OS time was not reached, and 8-year lymphoma-specific OS was 98.7%. Despite this, all POAML patients, including patients with Ann Arbor stage I disease, face a continuous risk of distant relapse. The risk of transformation to aggressive NHL was 4%, and CNS involvement was observed in 5 patients (3%).
Similar to published studies in the Western world, we observed that POAML is more common in females.3-6,22,23 A higher prevalence in males was only reported in studies originating from Asian countries like Japan15,24,25 and Korea.7,26-28 The gender difference seen in our series was due to a higher prevalence of conjunctival disease in females, with no difference at other locations. The median diagnosis age in our study was 63 years, which is similar to a previously reported median age at diagnosis of POAML ranging from 41 to 72 years.28-31 However, POAML can be seen in patients of any age, including children (the youngest patient in our cohort was 7 years old). We observed a higher incidence of preceding autoimmune disorders in POAML patients (12.4%) compared with previous reports (0% to 6%).5,32 However, in contrast to the etiological association between extranodal MALT lymphoma of the thyroid and salivary glands with Hashimoto thyroiditis and Sjogren syndrome, respectively, it is unlikely that the observed autoimmune processes directly contributed to POAML, since only a few of them involved the eyes or ocular adnexa. However, since B-cell receptors of POAML are autoreactive, local immune stimulation even without clinical autoimmune processes likely plays a role in the pathogenesis of these lymphomas.12 Further, local immune stimulation may also play an important role in patients with C psittaci infection.
The most common anatomical location of POAML was the orbital (56%), similar to most previous reports7,15 ; however, in some previous series, there was a similar frequency of orbital and conjunctival POAML.30,33 Only few studies demonstrated conjunctival involvement as the most common anatomical location of tumor.28,34 It is possible that there are some geographical changes in the anatomical predisposition of POAML. However, in our cohort as well as in previous studies, it was uncommon to observe POAML solitary localized to the eyelid. In our experience, eyelid involvement is usually caused by more aggressive and commonly disseminated lymphomas.
Bilateral involvement was observed in 15.6% of our patients, similar to the incidence reported in the literature (7% to 24%).15,24,25,27,31,34 Previous studies either did not analyze the association between bilateral involvement and clinical outcome or did not have significant statistical power to show such an association due to the small number of analyzed cases. Herein, we demonstrate that bilateral involvement is an independent clinical variable associated with inferior outcome in univariate analysis. Specifically, we show that patients with bilateral disease exhibit statistically shorter OS and PFS, with median TTP of 73 vs 161 months in patients with unilateral disease.
Similar to all previous reports, the majority of our patients presented with Ann Arbor stage I disease.3,4,30,33,35 Only 20% of our POAML patients presented with more advanced disease, and BM was involved in 10 patients (9%) who underwent BM biopsy. BM biopsy upgraded patients’ stage from Ann Arbor stage I to IV in only 6 cases. There were no differences in relapse rate, PFS, TTP, and OS between patients who did and did not have a staging BM biopsy. Similarly, freedom from progression and OS were not affected by BM involvement in patients with MALT lymphoma of other primary sites.36 The OS of patients with microscopic BM involvement was similar to that of patients without BM involvement. Therefore, we suggest that a BM biopsy may not be a necessary component of POAML staging.
All cases analyzed in our study were C psittaci negative. However, an association between POAML and C psittaci was reported in other geographic regions.8,9 Consequently, doxycycline treatment was studied as an initial therapeutic approach to POAML in several studies.37-39 The overall response rate ranges from 45% to 65% in most studies37,40 but was zero in a study reported by Grunberger et al.41 The 5-year PFS ranged from 55% to 61% in studies demonstrating response.38,42 Information on C psittaci infection was reported in only 3 of the 9 studies.38-40 Kim et al40 reported equal responses in patients with C psittaci–positive and negative POAML, while 2 trials from Italy showed better responses in C psittaci–positive patients.38,39 Only 1 of our patients received treatment with doxycycline with no response.
RT was reported to achieve an excellent local control (86% to 100%) in all previous studies of patients with Ann Arbor stage I POAML.14,25,34,43,44 Similarly, RT achieved local control in 97% of our Ann Arbor stage I POAML patients. However, there is still no consensus on the optimal dose of RT. The International Lymphoma Radiation Oncology Group guidelines for POAML recommend doses of 24 to 25 Gy in 1.5 to 2 Gy fractions to achieve high rates of local control with minimal toxicity.17 The National Comprehensive Cancer Network guidelines45 recommend doses of 24 to 30 Gy for low grade lymphomas. However, some studies report excellent outcomes with doses lower than 25 Gy31,45,46 while other studies showed good responses at higher doses of 36 Gy.25 Ejima et al24 and Son et al34 suggested doses of 30 to 30.6Gy. Fung et al,3 who analyzed 53 cases of MALT, reported 5-year local control rate of 81% vs 100% in patients treated with RT at doses of <30 Gy and ≥30 Gy, respectively (P < .01). Thus the literature reports effectiveness of a wide range of RT doses based mainly on small studies.
Herein, we demonstrate that RT at dose < 30.6 Gy is associated with statistically higher incidence of local and systemic relapse and shorter PFS compared with patients with a RT dose ≥30.6 Gy, but there is no advantage in OS. A similar observation was made by Fung et al.3 While patients treated with 30 Gy in 15 fractions and 30.6 Gy in 17 fraction receive a similar biologically equivalent dose (36 Gy), the dose per fraction and duration of therapy are slightly different and may be important for better control of this tumor. Overall, additional analyses performed by us demonstrate that even higher doses are associated with even better PFS but also with increased risk of local complications (data not shown). Because 24 Gy in 12 fractions results in a biologically equivalent dose of 28.8 Gy, a dose range of 24 to 30 Gy, as recommended by the guidelines, may be acceptable, but we use 30.6 Gy based on our experience. Doses <24 Gy can lead to local failure that may be observed only upon long periods of follow-up. In our series, we observed 4 local ipsilateral failures at doses of 30 Gy, 24 Gy, and 22 Gy each and an ipsilateral intraocular relapse following 24 Gy at 4 to 6 years of follow-up, except the patient receiving 22 Gy, who relapsed at 2 months. Ipsilateral failure was observed at 16.5 years of follow-up in a single patient who received RT at a dose of 30.6 Gy. The contradictory reports on effectiveness of different RT doses also most likely stem from the small number of patients with a limited power to show statistically significant differences in local control and did not analyze the impact of RT dose on PFS.13,14,16,34
Irrespective of the RT dose and despite an excellent initial clinical response to RT in patients with Ann Arbor stage I POAML, local and systemic relapses occurred continuously over time, with an estimated cumulative relapse or progression of 5.1% at 1 year, 17.5% at 5 years, and 31% at 10 years. Fung et al3 showed that in 39 cases with stage I MALT lymphoma, the freedom from distant relapse rate was 75% and 45% at 5 and 10 years, respectively. In a systematic review of POAML, Decaudin et al47 reported that systemic relapses were observed in 6% to 50% cases (mean 17%) and local relapses in up to 15% of patients treated with RT. This high rate of systemic relapse for Ann Arbor stage I POAML contrasts with relapse rates reported for localized MALT lymphoma of the stomach, which demonstrated a very low risk of distant recurrence but is concordant with the high risk of systemic relapse observed in other nongastric MALT lymphomas48,49 and may be attributed to the presence of microscopic disease outside the treated area. Of note, we observed 5 CNS relapses, an event rarely reported in the literature. We also observed transformation to DLBCL in 4% of patients, similar to other nongastric MALT lymphomas, as reported by Conconi et al.50 Interestingly, this study did not report transformation in patients with POAML.
Previous studies reported that advanced Ann Arbor disease stage, age >60 years, a nonconjunctival primary site, nodal involvement, presence of B symptoms, and elevated serum LDH levels were associated with inferior outcome of POAML patients.3,4,22,23,49 However, the negative prognostic value of many of these variables was not reproducible. The size of our cohort and long follow up allowed us to look for clinical parameters associated with distinct outcomes. Only age>60 years and bilateral involvement were associated with OS, but not with lymphoma-specific OS. Shorter PFS was associated with age >60 years, advanced Ann Arbor stage, bilateral disease, and radiation dose <30.6 Gy. The multivariate analysis confirmed independent prognostic power for shorter PFS for age >60 years and RT dose <30.6 Gy in unselected patients with POAML. Further, in Ann Arbor stage I patients, the multivariate analysis showed that age >60 and RT dose <30.6 Gy are also independently associated with shorter PFS. While we found significant correlation between shorter PFS and lower doses of radiation, we acknowledge that these findings are based on small number of cases treated with RT at lower doses. Further, our study is retrospective, encompassing patients treated over long period with variability in not only RT dose but also fractions and schedules, and these factors may introduce confounding variables that difficult to control for on multivariate analysis. These limitations preclude derivation of recommendations on the dose of RT, but indicate the urgent need for prospective randomized trial that should address this question.
In summary, we confirm the indolent nature of POAML, characterized by long survival and low lymphoma-attributed mortality. However, we also show that POAMLs harbor a persistent risk of relapses, including in the CNS, and a risk of transformation to aggressive lymphoma. Therefore, long-term follow-up is required for these patients. We demonstrate that BM involvement does not affect outcome of POAML patients and suggest that it is not a necessary component of initial staging. These findings will be useful for comparison and stratification of POAML patients in clinical studies and planning future treatment studies in these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carol Karp from the Bascom Palmer Eye Institute for her contribution of clinical cases to this article.
I.S.L. is supported by the American Society of Hematology Bridge grant; the Dwoskin, Recio and Anthony Rizzo Families Foundations; and the University of Miami Sylvester Comprehensive Cancer Center.
Authorship
Contribution: A.D. conceptualized and designed the study, collected and analyzed the data, and wrote the manuscript; M.G.J. collected and analyzed the data; L.L. collected the data; J.R.C. performed review of diagnostic biopsies and confirmed diagnosis; F.V. performed review of diagnostic biopsies and confirmed diagnosis; R.T. analyzed the data; D.T. analyzed the data and was involved in treatment of these patients; A.M. analyzed the data and was involved in treatment of these patients; I.S.L. conceptualized and designed the study, analyzed the data, and wrote the paper; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, Division of Hematology and Oncology, Sylvester Comprehensive Cancer Center, University of Miami, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: ilossos@med.miami.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal