To the editor:
The immune response to complexes of the chemokine platelet factor 4 (PF4) and polyanions1,2 results in anti–PF4/polyanion (anti–PF4/P) antibodies, which can induce one of the most frequent immune-mediated adverse drug reactions: heparin-induced thrombocytopenia (HIT). Immune complexes composed of anti–PF4/P antibodies and PF4/P complexes on the platelet surface induce platelet aggregation via cross-linking FcγRIIA receptors.3,4 They also bind to the surface of endothelial cells and monocytes,5-7 inducing procoagulant activity.5,8 Of the various polyanions that can form complexes with PF4, PF4/heparin (PF4/H) complexes have been most studied.
KKO is a monoclonal antibody that mimics human anti–PF4/P antibodies, inducing HIT, and activates platelets in vitro and in vivo.9 KKO has been used to unravel the pathogenesis of HIT and is the basis for a recent US Food and Drug Administration–approved plasma-based antigen assay (HemosIL HIT-Ab[PF4-H]) for detection of anti–PF4/P antibodies.10,11 More recently, the crystal structure of the PF4 tetramer/KKO-Fab complex has been characterized, in which an epitope on an “open” end of the PF4 tetramer was stabilized by fondaparinux to allow binding of KKO.12
Recently, we found by single-molecule force spectroscopy that human anti–PF4/P antibodies are qualitatively different; that is, platelet-activating (potentially clinically relevant) anti–PF4/P antibodies interact with PF4/H complexes on a solid phase (gold surface) with much higher binding forces than nonactivating antibodies.13 In contrast, KKO, which has characteristics similar to those of clinically relevant, platelet-activating human anti–PF4/P antibodies and strongly induces platelet aggregation in the presence of PF4 and heparin or PF4/P complexes (supplemental Figure 1, available on the Blood Web site), shows only weak binding forces to purified PF4/H complexes.13
We hypothesized that KKO might bind to PF4/H complexes in the purified solid-phase system differently than PF4/H complexes coated on the platelet surface. It is well known that the epitope for anti–PF4/P antibodies is conformation sensitive, and additional polyanions on the platelet surface (such as chondroitin sulfate or polyphosphates)14,15 might influence the conformation of PF4 and PF4/H complexes15 or their three-dimensional presentation. To further investigate our hypothesis, we combined biophysical and immunohematological methods and directly compared the binding forces of KKO on a single-molecule level when it interacted with PF4 or PF4/H complexes immobilized either on a solid phase (purified system) or on platelet surfaces.
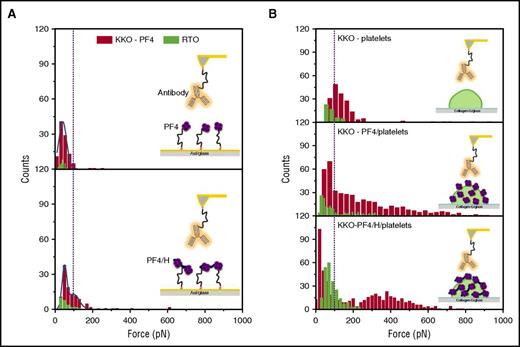
We immobilized KKO on an atomic force microscope (AFM) cantilever and approached it to PF4 or PF4/H complexes coated either on a gold surface or on platelets for interaction utilizing a single-molecule force spectroscopy technique (Figure 1) (see supplemental Methods for details). The binding forces between KKO and PF4 or PF4/H complexes were obtained when AFM-cantilevers detached from the surfaces. As a control, we used RTO (a murine monoclonal antibody that binds PF4 or PF4/H complexes but does not induce platelet aggregation).16 All experiments on the platelet surface were performed in the presence of the blocking monoclonal antibody IV.3 to exclude any interaction of KKO or RTO with platelet FcγRIIa receptors.4 We found major differences in the binding forces between KKO binding to PF4 in the solid-phase system and on the platelet surface.
The binding strength of KKO (red) vs RTO (green) to PF4 or PF4/H complexes coated on different surfaces. (A) When PF4 (top) or PF4/H complexes (bottom) were immobilized on a gold surface, the reaction between KKO (red) and PF4 is weaker (rupture force up to 100 pN) than that between KKO and PF4/H complexes (rupture force up to 200 pN), while RTO (green) shows much weaker interactions, as evidenced by the low binding “counts.” (B) When PF4 (middle) or PF4/H complexes (bottom) were coated on platelet surfaces, both KKO (red; rupture force up to 800 pN) and RTO (green; rupture force up to 200 pN) show stronger interaction forces than on the solid phase (A) or on noncoated platelets (top). The broad distribution of binding forces indicates that the binding site of KKO is presented very variably, allowing weak to very strong binding of KKO.
The binding strength of KKO (red) vs RTO (green) to PF4 or PF4/H complexes coated on different surfaces. (A) When PF4 (top) or PF4/H complexes (bottom) were immobilized on a gold surface, the reaction between KKO (red) and PF4 is weaker (rupture force up to 100 pN) than that between KKO and PF4/H complexes (rupture force up to 200 pN), while RTO (green) shows much weaker interactions, as evidenced by the low binding “counts.” (B) When PF4 (middle) or PF4/H complexes (bottom) were coated on platelet surfaces, both KKO (red; rupture force up to 800 pN) and RTO (green; rupture force up to 200 pN) show stronger interaction forces than on the solid phase (A) or on noncoated platelets (top). The broad distribution of binding forces indicates that the binding site of KKO is presented very variably, allowing weak to very strong binding of KKO.
In the solid-phase system (Figure 1A), the binding forces of RTO (green) and KKO (red) when interacting with 100 µg/mL PF4 coated on the gold surface did not differ substantially (36.3 ± 1.4 pN vs 41.3 ± 0.8 pN). However, binding occurred less frequently with RTO (∼3% frequency of rupture events) than with KKO (∼11% frequency of rupture events), indicating a lower affinity of RTO, consistent with previous studies.16,17 KKO interacted with PF4/H complexes immobilized on a gold surface with binding forces up to 200 pN, which were slightly stronger than the forces when KKO interacted with PF4 (up to 100 pN; P < .001). Binding of RTO was weak and occurred less frequently compared with KKO (Figure 1A).
For both KKO and RTO, the binding events increased when PF4 or PF4/H complexes were immobilized on the platelet surface compared with the solid-phase system (Figure 1). On the platelet surface (Figure 1B), the rupture force of KKO increased ∼4-fold (compared with the gold surface) when it bound to PF4 or PF4/H complexes (up to 800 pN). Binding of KKO did not increase substantially when platelets were precoated with 25 µg/mL PF4 (Figure 2C). When tested with platelets not preincubated with PF4, the interaction forces of KKO were much weaker. Most probably, KKO interacted only with some PF4 bound to glycosaminoglycans on the platelet surfaces. Also, RTO bound weakly to washed platelets not precoated with PF4. The binding force of RTO to PF4 (up to 100 pN) or PF4/H complexes (up to 200 pN) on the platelet surface was higher than that in the solid-phase system, but it increased much less compared with that of KKO.
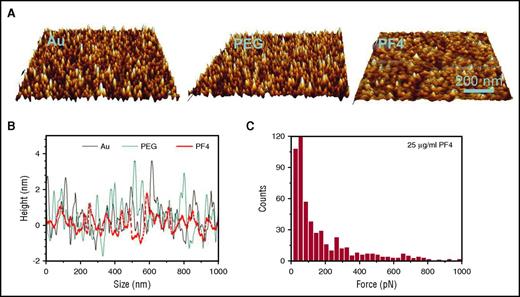
Control experiments for controlling PF4 density coated on the gold surface and on platelets. (A-B) AFM images of gold surface (A, left), polyethylene glycol (PEG)–coated gold surface (A, middle), and PF4-coated PEG/gold surface (A, right) show no significant differences in roughness between gold surface before (B, black) and after coating with PEG (B, blue) but reduced roughness after coating with PF4 (B, red). This illustrates that PF4 molecules covered the gold surface in high density and excludes that the reduced binding force of KKO in the solid-phase system is due to suboptimal antigen density. (C) The binding strength of KKO to 25 µg/mL PF4-coated platelets shows a force profile similar to that obtained on platelets coated with 10 µg/mL PF4 (Figure 1B, middle). Images in panels A and B were modified from Nguyen et al18 with permission.
Control experiments for controlling PF4 density coated on the gold surface and on platelets. (A-B) AFM images of gold surface (A, left), polyethylene glycol (PEG)–coated gold surface (A, middle), and PF4-coated PEG/gold surface (A, right) show no significant differences in roughness between gold surface before (B, black) and after coating with PEG (B, blue) but reduced roughness after coating with PF4 (B, red). This illustrates that PF4 molecules covered the gold surface in high density and excludes that the reduced binding force of KKO in the solid-phase system is due to suboptimal antigen density. (C) The binding strength of KKO to 25 µg/mL PF4-coated platelets shows a force profile similar to that obtained on platelets coated with 10 µg/mL PF4 (Figure 1B, middle). Images in panels A and B were modified from Nguyen et al18 with permission.
As the interaction of antibody and antigen directly depends on the optimal presentation of the epitope, the different binding forces of KKO strongly indicate that PF4 and PF4/H complexes either expose different epitopes or allow better access of this monoclonal antibody to its epitope when bound to the platelet surface compared with their presentation on an inert gold surface. This further emphasizes that PF4/H complexes present the antigenic site differently when interacting with binding partners on platelet surfaces,14 and it provides an explanation for our surprising observation that KKO showed relatively weak reactivity when tested with PF4/H complexes in the solid-phase system,13 though at the same time, it strongly activates platelets in functional assays. However, we cannot exclude that besides a conformational change in PF4, the presentation of PF4/H complexes in a three-dimensional pattern on the platelet surface also contributes to better antibody binding. Currently, it is unresolved which additional binding partners on platelet surfaces interfere with the conformational change or different presentations of PF4/H complexes. However, chondroitin sulfate14 and polyphosphates15 are potential candidates. To exclude that the density of PF4 or PF4/H complexes on the gold surface was too low to allow both Fab moieties of KKO to bind, we tested coverage of the gold surface with PF4 by AFM and found a fully covered surface (Figure 2A-B).18
These results were obtained by the model of monoclonal antibodies, which allows highly controlled experimental conditions. Transferred to human antibodies, our findings suggest that the same mechanism might be the underlying reason why a subset of human anti–PF4/H antibodies binds weakly in antigen assays but still strongly activates platelets in functional assays and causes HIT in vivo.19 It is recommended that a positive PF4/heparin enzyme immunoassay should prompt confirmatory testing by serotonin release assay20 or heparin-induced platelet activation.1 However, these functional assays are only available in specialized laboratories, and in many countries, no functional test for platelet-activating anti–PF4/P antibodies is available. Therefore, many physicians rely on the results of antigen tests, especially for the first days after clinical suspicion of HIT has been raised, until the results of the functional assay is reported (or they depend entirely on the results of the antigen test). As ∼50% of anti–PF4/H antibodies detected by antigen tests are clinically irrelevant, it had been suggested to use higher optical density (OD) cutoffs of the antigen tests (eg, OD ≥1.0) to increase the specificity for clinically relevant antibodies.21 Until further studies with human anti–PF4/P antibodies are performed, our study raises a note of caution to use higher cutoffs in antigen tests21 and to not exclude HIT in case of low reactivity in the antigen test (eg, enzyme immunoassay OD <1.0) if the clinical presentation is highly suggestive for HIT.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Ulrike Strobel and Ricarda Raschke for providing washed platelets and help with heparin-induced platelet activation experiments and Nikolay Medvedev with the help with scanning electron microscopy images.
This work was supported by the Deutsche Forschungsgemeinschaft (Germany) (NG 133/1-1).
Contribution: T.-H.N. designed and performed the experiments, analyzed and interpreted the data, and wrote the manuscript; and A.G. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Greinacher, Institute for Immunology and Transfusion Medicine, University Medicine Greifswald, 17475 Greifswald, Germany; e-mail: greinach@uni-greifswald.de; and Thi-Huong Nguyen, Institute for Immunology and Transfusion Medicine, University Medicine Greifswald, 17475 Greifswald, Germany; e-mail: nguyent@uni-greifswald.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal