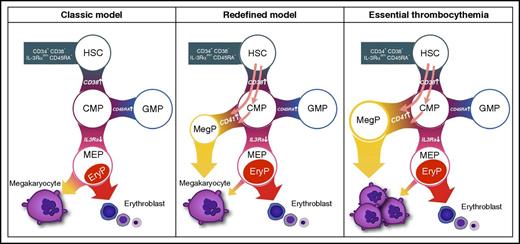
Model of megakaryopoiesis proposed by Miyawaki et al. Megakaryocyte production in human bone marrow proceeds through a unilineage MegP progenitor that can be purified within the classic CMP compartment as CD34+ CD38+ CD45RA− IL-3Rαdim CD41+. In patients with JAK2 V617F mutated essential thrombocythemia, the MegP population is expanded and may contribute to disease pathology. EryP, unipotent erythrocyte progenitor; GMP, granulocyte-macrophage progenitor. The figure has been adapted from Figure 7 in the article by Miyawaki et al that begins on page 3332.
Model of megakaryopoiesis proposed by Miyawaki et al. Megakaryocyte production in human bone marrow proceeds through a unilineage MegP progenitor that can be purified within the classic CMP compartment as CD34+ CD38+ CD45RA− IL-3Rαdim CD41+. In patients with JAK2 V617F mutated essential thrombocythemia, the MegP population is expanded and may contribute to disease pathology. EryP, unipotent erythrocyte progenitor; GMP, granulocyte-macrophage progenitor. The figure has been adapted from Figure 7 in the article by Miyawaki et al that begins on page 3332.
Here, Miyawaki et al focused on the human myeloid progenitor compartment and used a combination of single-cell quantitative polymerase chain reaction and in vitro differentiation assays to identify a homogeneous population of cells, which fate has restricted to megakaryopoiesis. For this, they investigated single cells from the classically defined CMP, MEP, and granulocyte-macrophage progenitor compartments found in the CD34+ CD38+ fraction of human bone marrow. The only cells that were uniquely committed to produce megakaryocytes in vitro and in vivo (unipotent megakaryocyte progenitors [MegPs]) were found as a distinct subpopulation within the CMP compartment (see figure). This small population (∼8% of CMPs) was marked by CD41 cell surface expression and characterized by high expression of the megakaryocyte master transcription factor Fli1. In contrast, only a small percentage of single MEPs would give rise to small megakaryocytic colonies. Interestingly, Miyawaki et al showed that the MegP population was found both in umbilical cord blood and adult bone marrow, suggesting that its presence is maintained over a human lifetime. Of note, CD41− CMPs could also produce robust platelet engraftment in vivo but always in combination with myeloid and erythroid cells. The key message from this work is that fate restriction to megakaryocyte production predominantly occurs at the level of the CD41+ MegP population in human bone marrow.
A handful of recent studies used single-cell tools to identify when erythroid and megakaryocyte potential separate in humans. Two of these have reported the existence of small pockets of bilineage erythroid-megakaryocyte progenitor cells within the CMP5 and MEP populations,5,6 but no unilineage MegPs. The discrepancy between these studies and that of Miyawaki et al is likely to be largely technical, because different sorting strategies and/or cellular sources were used. In addition, genome-wide single-cell RNA-sequencing of the whole bone marrow CD34+ compartment showed strong unilineage megakaryocyte priming in a fraction of the CD34+ CD38+ compartment and the existence of a subpopulation of multipotent progenitors with high contribution to megakaryopoiesis.7 What emerges is a picture in which, at the single-cell level, the classically defined CMP and MEP are much more heterogeneous than originally thought. Specifically, it seems that the proportion of cells that have capacity to differentiate into more than one mature blood cell type is very likely low, especially in adults. Similar conclusions were recently reached in mouse models.8,9
Two questions that remain open are: How are MegPs specified at the molecular level? And which cell is their direct parent? The authors demonstrated that CD41− CMPs could give rise to CD41+ CMPs in vitro, but whether this is the main route of MegP production in vivo at homeostasis or following injury remains to be clarified. This may be difficult to assess directly in human cells with the current tools, but, because studies in mice have suggested that in certain conditions HSCs exclusively give rise to platelets2,4 and that megakaryocyte-restricted progenitors can reside in the compartment that is commonly used to purify HSCs,3,10 it would be interesting to know if the MegP is an obligatory cellular step during megakaryopoiesis.
Another important finding of this study is that MegPs isolated from patients with JAK2 V617F mutant ET are expanded and tend to produce more megakaryocytes than those from healthy controls (see figure). Direct comparison of the function of JAK2 V617F mutated and wild-type MegPs and their progenitors within the same patient was not carried out. However, one implication of these data is that mutations such as JAK2 V617F, recurrent in myeloproliferative neoplasms or in clonal hematopoiesis of indeterminate potential, can have strong effects not only on the HSC compartment but also on specific progenitor cell types. In the case of expansion of MegPs in ET, future studies will have to address how much this expansion accounts for ET pathology and identify which molecular mechanisms downstream of the JAK2 V617F mutation drive this expansion specifically in patients with ET and not in patients with other types of myeloproliferative neoplasms. A deep understanding of the normal routes of commitment, their fluctuations over a human lifetime, and how they are skewed in malignancy, will be highly informative for how we should treat blood diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal