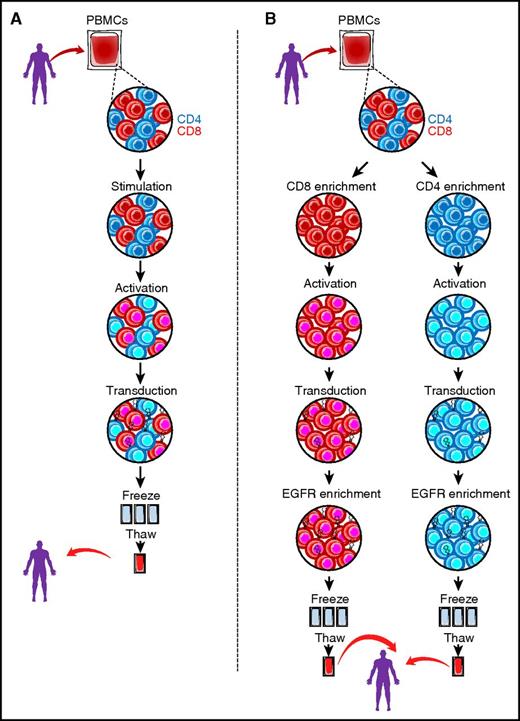
(A) Standard approach to manufacturing CD19 CAR T cells. After apheresis, bulk peripheral blood mononuclear cells (PBMCs) undergo stimulation and activation (with CD3/CD28 beads and cytokine supplementation) followed by transduction with a CD19 CAR vector of choice. After expansion, the bulk product is cryopreserved until ready for thaw and clinical use. (B) Manufacturing schema of PLAT-02 (defined formulation) CD19 CAR product. After apheresis, PBMCs undergo positive selection for CD8+ and CD4+ T cells by using immunomagnetic separation (CliniMACS device, Miltenyi Biotec). After enrichment, CD4 and CD8 T cells are separately activated with CD3/CD28 beads and then transduced with the lentiviral CD19 CAR vector expressing EGFRt in the presence of IL-7/IL-15 (CD4) and IL-15/IL-2 (CD8). The separate cell products undergo positive selection for EGFRt-expressing cells using the CliniMACS device, after which the individual products are cryopreserved until ready for thaw and clinical use.
(A) Standard approach to manufacturing CD19 CAR T cells. After apheresis, bulk peripheral blood mononuclear cells (PBMCs) undergo stimulation and activation (with CD3/CD28 beads and cytokine supplementation) followed by transduction with a CD19 CAR vector of choice. After expansion, the bulk product is cryopreserved until ready for thaw and clinical use. (B) Manufacturing schema of PLAT-02 (defined formulation) CD19 CAR product. After apheresis, PBMCs undergo positive selection for CD8+ and CD4+ T cells by using immunomagnetic separation (CliniMACS device, Miltenyi Biotec). After enrichment, CD4 and CD8 T cells are separately activated with CD3/CD28 beads and then transduced with the lentiviral CD19 CAR vector expressing EGFRt in the presence of IL-7/IL-15 (CD4) and IL-15/IL-2 (CD8). The separate cell products undergo positive selection for EGFRt-expressing cells using the CliniMACS device, after which the individual products are cryopreserved until ready for thaw and clinical use.
The authors report successful product release in 93% of enrolled patients and an overall intent-to-treat (ITT) minimal residual disease–negative (MRD–) remission rate of 89%. Of note, 100% of patients who received cyclophosphamide-fludarabine lymphodepletion had an MRD– remission, further reinforcing the importance of lymphodepletion regimens that include fludarabine, as opposed to cyclophosphamide alone.2 There were no deaths, and the adverse effect profile of the reported T-cell product was similar to or better than that in other published studies of CD19 CAR T cells, with ∼23% of patients experiencing severe cytokine release syndrome (CRS) and/or reversible severe neurotoxicity attributed to the T-cell product.3,4 With longer follow-up, the 12-month event-free survival was 50.8% and overall survival was 69.5%, which are similar to response rates reported in previous CD19 CAR T-cell studies from the University of Pennsylvania3 and the National Cancer Institute4 targeting ALL in pediatric patients. Of 18 relapses, 7 were associated with loss of CD19 expression, whereas loss of functional CAR T cells was a risk factor for CD19+ relapse, an incidence rate similar to that reported in other studies of CD19 CAR T cells.3,4
In this study, the investigators used a manufacturing process that resulted in CAR T-cell products with a defined CD4/CD8 composition, uniform high-level transgene expression, and attenuated differentiation. To achieve this, the investigators isolated CD4+ and CD8+ T cells from apheresis products by immunomagnetic separation. After enrichment, a defined number of CD4 and CD8 selected T cells were separately stimulated with CD3/CD28 beads, transduced with the lentiviral CD19 CAR vector also expressing EGFRt, and cultured with homeostatic cytokines to limit activation-induced differentiation. CD4 cultures were supplemented with interleukin-7 (IL-7) and IL-15, and CD8 cultures were supplemented with IL-15 and IL-2. Finally, CD3/CD28 bead particles were removed, and cultures were positively selected for EGFRt-expressing cells by using the CliniMACS device. The figure illustrates the more standardized CAR T-cell manufacturing approach (panel A) whereby CAR T cells are generated from bulk populations of T cells that typically lack the aforementioned selection and enrichment steps; panel B illustrates the manufacturing process used in the Gardner et al study. The rationale for this strategy comes from preclinical studies that suggest that a 1:1 ratio of CD4 to CD8 and culture with appropriate homeostatic cytokines would ensure maximum effectiveness of both T-cell subsets and would yield a less terminally differentiated more memory-prone T-cell population with maximum killing capacity, prolonged persistence, and the ability to retain memory and self-renewal capacity.5
Gardner et al directly attribute the very impressive ITT MRD– remission rates and overall survival achieved in their study to their T-cell product. A number of strategies have been explored to further enhance the efficacy of CAR T cells, such as the use of different costimulatory moieties (typically CD28 or 41BB) to provide enhanced T-cell activation and persistence6 and modification of the spacer or hinge regions of the construct.7,8 The impact of the cytokine milieu in which cells are manufactured and the most efficient T-cell phenotypes for CAR transduction and T-cell manufacture, as outlined in the Gardner et al article and elsewhere9 are additional important variables. To complicate matters, patient-specific characteristics such as age, absolute lymphocyte count (ALC), and previous therapy can also contribute to the quality of the T-cell product. Nevertheless, CD19 CAR T cells derived from different manufacturing techniques, different lentiviral or retroviral constructs, and different costimulatory moieties have had consistently excellent outcomes in studies targeting children or young adults with ALL.1,3,4 Thus, discerning which factors are most important for CD19 CAR T-cell persistence and antitumor activity in patients is a challenge. At present, it is not possible to confirm the contention of Gardner et al and thereby justify the more complex and expensive manufacturing process they used, based on patient response rates and survival. Longer-term follow-up on more patients will be necessary to determine whether there is a difference in long-term survival between different CD19 CAR T-cell products.
Another important finding in the article by Gardner et al is the suboptimal expansion and persistence of CD19 CAR T cells in patients with MRD who have low quantities of normal and malignant CD19+ B cells. As the authors point out, lack of the targeted antigen could certainly present a challenge when attempting to incorporate CAR T-cell therapy into first-line therapy or as treatment of MRD after induction or consolidation. Additional means of addressing this potential shortcoming are being explored by several groups and include targeting multiple antigens with CAR T cells or providing additional antigen as a vaccine with CAR T-cell infusion.
Although the multistep manufacturing process used in the Gardner et al study is more complex, it is notable that they report a high success rate in manufacturing with that process, and it is commendable that they report their results on ITT analysis. In some previous studies, it has proved difficult to decipher how many patients were not eligible on the basis of ALC or failure of a test culture.10 As CD19 CAR T cells move toward licensure, it will be important to streamline the process to reduce the cost of goods and also to determine the standardized ITT response rates with all products to definitively learn whether additional manufacturing maneuvers such as those used by Gardner et al are beneficial. It is an intriguing possibility that a major benefit of the initial separation of the CD4 and CD8 T cells and their independent growth in optimized homeostatic cytokines may be in increasing the manufacturing success rate and making CD19 CAR T-cell therapy available to a greater percentage of patients.
Conflict-of-interest disclosure: R.H.R. is a member of the Novartis Treatment Advisory Landscape Board and received honoraria. H.E.H received research support from Cell Medica and Celgene and is a founder of ViraCyte.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal