Abstract
Thrombotic thrombocytopenic purpura (TTP) is a rare and life-threatening thrombotic microangiopathy characterized by microangiopathic hemolytic anemia, severe thrombocytopenia, and organ ischemia linked to disseminated microvascular platelet rich-thrombi. TTP is specifically related to a severe deficiency in ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13), the specific von Willebrand factor-cleaving protease. ADAMTS13 deficiency is most frequently acquired via ADAMTS13 autoantibodies, but rarely, it is inherited via mutations of the ADAMTS13 gene. The first acute episode of TTP usually occurs during adulthood, with a predominant anti-ADAMTS13 autoimmune etiology. In rare cases, however, TTP begins as soon as childhood, with frequent inherited forms. TTP is ∼2-fold more frequent in women, and its outcome is characterized by a relapsing tendency. Rapid recognition of TTP is crucial to initiate appropriate treatment. The first-line therapy for acute TTP is based on daily therapeutic plasma exchange supplying deficient ADAMTS13, with or without steroids. Additional immune modulators targeting ADAMTS13 autoantibodies are mainly based on steroids and the humanized anti-CD20 monoclonal antibody rituximab. In refractory or unresponsive TTP, more intensive therapies including twice-daily plasma exchange; pulses of cyclophosphamide, vincristine, or cyclosporine A; or salvage splenectomy are considered. New drugs including N-acetylcysteine, bortezomib, recombinant ADAMTS13, and caplacizumab show promise in the management of TTP. Also, long-term follow-up of patients with TTP is crucial to identify the occurrence of other autoimmune diseases, to control relapses, and to evaluate psychophysical sequelae. Further development of both patients’ registries worldwide and innovative drugs is still needed to improve TTP management.
Introduction
The last 20 years have been marked by the connection between an old disease, the thrombotic thrombocytopenic purpura (TTP),1 and a young protein, ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13).2 Based on better understanding of pathophysiology and as a result of the creation of TTP registries worldwide, major advances in the comprehension of this historically fatal disease related to widespread microvascular platelet thrombi have been made, allowing a significant improvement in both diagnosis and therapeutic management.3-10
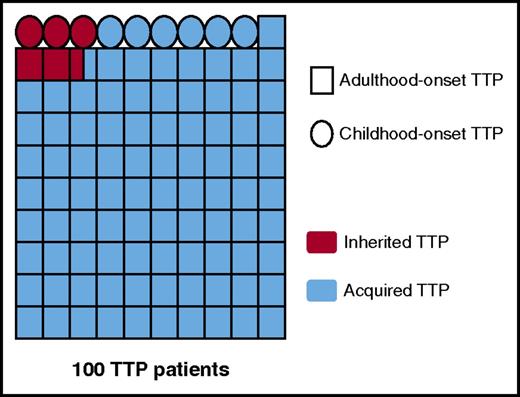
Today, TTP is well established as a rare hematologic disease with an average annual prevalence of ∼10 cases/million people and an annual incidence of ∼1 new case/million people.10,11 The first acute episode of TTP mostly occurs during adulthood (∼90% of all TTP cases), but some child- and adolescent-onset forms also exist (∼10% of all TTP cases; Figure 1).3,5-10,12,13 TTP is mainly caused by an autoimmune mechanism, but rare nonimmune inherited forms are described (Upshaw-Schulman syndrome [USS] OMIM 604.134; Figure 1). TTP is ∼2-fold more frequent in women, and its clinical course is characterized by a relapsing tendency. TTP remains a life-threatening disease with a mortality rate of 10% to 20% in spite of appropriate therapeutic management.14
TTP as a function of age of onset and mechanism for ADAMTS13 deficiency. The proportions of adulthood-/childhood-onset TTP and acquired/inherited TTP, respectively, presented in this figure were calculated from the data of the French Registry for TTP (840 patients).10,13 These data are in agreement with miscellaneous demographic data reported in the literature by other teams.3,5-9 The diagram shows 100 patients with TTP, each patient being represented by a symbol, either a square for patients with adulthood-onset TTP (91%) or a circle for patients with childhood-onset TTP (9%). Acquired TTP is presented in blue (94.5%), and inherited TTP (USS) in red (5.5%). Interestingly, the proportion of USS is very low (2.5%) in adulthood-onset TTP, whereas it is as high as 33% in childhood-onset USS.
TTP as a function of age of onset and mechanism for ADAMTS13 deficiency. The proportions of adulthood-/childhood-onset TTP and acquired/inherited TTP, respectively, presented in this figure were calculated from the data of the French Registry for TTP (840 patients).10,13 These data are in agreement with miscellaneous demographic data reported in the literature by other teams.3,5-9 The diagram shows 100 patients with TTP, each patient being represented by a symbol, either a square for patients with adulthood-onset TTP (91%) or a circle for patients with childhood-onset TTP (9%). Acquired TTP is presented in blue (94.5%), and inherited TTP (USS) in red (5.5%). Interestingly, the proportion of USS is very low (2.5%) in adulthood-onset TTP, whereas it is as high as 33% in childhood-onset USS.
Historical milestones for TTP understanding
In 1924, TTP was first clinically described by Eli Moschcowitz in a 16-year-old girl as a fatal thrombotic microangiopathy (TMA) including weakness, fever, transient focal neurologic symptoms, severe thrombocytopenia, and microangiopathic hemolytic anemia linked to the presence of systemic visceral microthrombosis of the terminal arterioles and capillaries.1 Until the 1980s to 1990s, the etiology for TTP remained unknown, and its outcome was fatal in ∼90% of cases. In 1982, the role of von Willebrand factor (VWF), a multimeric glycoprotein crucial for platelet adhesion and aggregation at high shear rates of blood flow, was first suspected because patients with TTP exhibited plasma ultralarge VWF multimers hyperadhesive to platelets.15 In 1985, the presence of large amounts of VWF (when compared with fibrinogen) was demonstrated within the visceral platelet microthrombi identified by histopathology in a decedent patient with TTP.16 Empirical therapeutic plasma exchange (TPE) was shown to dramatically improve TTP prognosis, allowing an 85% survival.17 This suggested a deficiency of a plasma protein able to regulate VWF multimer size was responsible for the disorder. In 1996, a novel metalloprotease (VWF-CP for VWF cleaving-protease) able to specifically cleave VWF was purified from human plasma.18 In 1998, a severe functional deficiency of the VWF-CP, either congenital or acquired via specific autoantibodies, was demonstrated to be responsible for TTP.19,20 In 2001, as a result of both a gene cloning approach2 and a protein-sequencing analysis,21 the VWF-CP was identified as ADAMTS13, the 13th member of the ADAMTS protein family. Since 2001, several subsequent studies dedicated to patients with miscellaneous TMA have shown that ADAMTS13 activity less than 10% is specific for TTP.22-24
Definition and pathophysiology
The definition for TTP has changed over time. Initially, an acute episode of TTP was defined by clinical criteria (multivisceral ischemic symptoms mainly targeting the brain) and standard biology criteria (microangiopathic hemolytic anemia and severe thrombocytopenia) occurring in the absence of other apparent causes. This definition was recently completed by the presence of a severe deficiency of ADAMTS13 (activity <10%), which is the only biologic marker specific for TTP.14
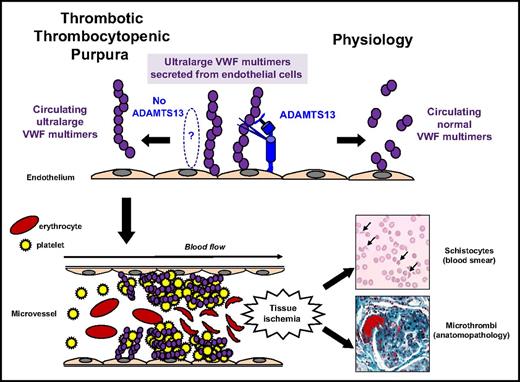
A severe functional ADAMTS13 deficiency causes the blood accumulation of platelet-hyperadhesive ultralarge VWF multimers, leading to the formation of platelet-rich microthrombi within small arterioles (Figure 2).14 In most cases, the mechanism for ADAMTS13 severe deficiency is acquired via autoantibodies to ADAMTS13, as demonstrated by positive anti-ADAMTS13 IgG in ∼75% of TTP during an acute phase (Figure 3).10,14 These anti-ADAMTS13 IgGs usually inhibit the proteolytic activity of ADAMTS13 toward VWF, and a significant amount of circulating ADAMTS13-specific immune complexes (ICs) have been reported in acquired forms of TTP. These mechanisms both contribute to ADAMTS13 severe deficiency.25 Also, ADAMTS13 autoantibodies are polyclonal with epitopes targeting the Cystein-rich/spacer domain of ADAMTS13.26 Anti-ADAMTS13 IgM and IgA systematically associated with anti-ADAMTS13 IgG were rarely reported in acute TTP.27 Also, anti-ADAMTS13 IgG may not be detectable in ∼20% to 25% of acute TTP, raising the hypothesis that severe ADAMTS13 deficiency in these patients may result from different, as-yet-unclear mechanisms (Figure 3).10,14 Several hypothesis may explain ADAMTS13 severe deficiency in these cases: lack of sensitivity of anti-ADAMTS13 IgG assays in detecting IgG trapped within immune complexes28 ; involvement of other Ig isotypes27 ; decreased synthesis/secretion of ADAMTS13 (ie, in acute liver insufficiency)29 ; degradation of ADAMTS13 by sepsis enzymes such as calpains, elastases, thrombin, or plasmin30-32 ; and catalytic inhibition of ADAMTS13 by free hemoglobin or interleukines.32
Pathophysiology for TTP. In physiologic conditions, ultralarge VWF multimers released from endothelial cells are cleaved by ADAMTS13 in smaller VWF multimers, less adhesive to platelets. In TTP, because of the absence of functional ADAMTS13 (either absent by congenital defect or inhibited by specific autoantibodies), ultralarge VWF multimers are released into the blood and bind spontaneously to platelets to form aggregates within the arterial and capillary microvessels. The VWF–platelet aggregates are large enough to form microthrombi inducing tissue ischemia, platelet consumption, and microangiopathic hemolytic anemia (schistocytes on blood smear).
Pathophysiology for TTP. In physiologic conditions, ultralarge VWF multimers released from endothelial cells are cleaved by ADAMTS13 in smaller VWF multimers, less adhesive to platelets. In TTP, because of the absence of functional ADAMTS13 (either absent by congenital defect or inhibited by specific autoantibodies), ultralarge VWF multimers are released into the blood and bind spontaneously to platelets to form aggregates within the arterial and capillary microvessels. The VWF–platelet aggregates are large enough to form microthrombi inducing tissue ischemia, platelet consumption, and microangiopathic hemolytic anemia (schistocytes on blood smear).
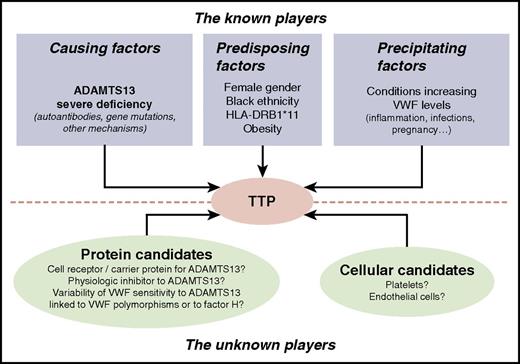
Known and unknown players involved in TTP. Among the known players involved in TTP occurrence, ADAMTS13 severe deficiency, either acquired via specific autoantibodies or inherited via ADAMTS13 gene mutations, is the only causing factor identified so far. Other factors are well established as predisposing factors for acquired TTP (ie, female sex, black ethnicity, HLA-DRB1*11, and obesity). Also, pathophysiological conditions increasing plasma VWF levels such as inflammation, sepsis, or pregnancy are known to potentially act as precipitating factors of acute episodes of either acquired or inherited TTP. Other still unknown players are suspected to be involved in TTP occurrence: these may be either proteins of the ADAMTS13/VWF system or cellular candidates such as platelets or endothelial cells.
Known and unknown players involved in TTP. Among the known players involved in TTP occurrence, ADAMTS13 severe deficiency, either acquired via specific autoantibodies or inherited via ADAMTS13 gene mutations, is the only causing factor identified so far. Other factors are well established as predisposing factors for acquired TTP (ie, female sex, black ethnicity, HLA-DRB1*11, and obesity). Also, pathophysiological conditions increasing plasma VWF levels such as inflammation, sepsis, or pregnancy are known to potentially act as precipitating factors of acute episodes of either acquired or inherited TTP. Other still unknown players are suspected to be involved in TTP occurrence: these may be either proteins of the ADAMTS13/VWF system or cellular candidates such as platelets or endothelial cells.
A last, and rare (∼2% of all cases), mechanism for ADAMTS13 severe deficiency is genetic via recessively inherited biallelic mutations of the ADAMTS13 gene (patients are either homozygous or compound heterozygous), causing congenital TTP or USS (Figure 3).2 About 150 distinct mutations of ADAMTS13 gene are reported worldwide, with a low degree of overlap between the continents.33 These mutations are spread all over the ADAMTS13 gene, with most of them being located within the N-terminal region; they consist of ∼70% of missense mutations and ∼30% of truncating mutations (no total deletion of ADAMTS13 gene has ever been described). Characterization of recombinant mutated ADAMTS13 proteins has demonstrated that ADAMTS13 gene mutations are mostly responsible for quantitative ADAMTS13 defects.34 The clinical penetrance of ADAMTS13 mutations may be variable. The numerous mutations identified in childhood-onset USS are distinct from those found in adulthood-onset USS, characterized by the high frequency of 1 specific mutation, p.Arg1060Trp.10,35,36 These autoimmune and genetic etiologies for TTP have been proven in mouse and baboon animal models, respectively.37,38
Severe ADAMTS13 deficiency is the only causing factor for TTP identified so far. Although it is necessary to cause TTP, deficiency of enzyme activity is not sufficient on its own to induce the clinical syndrome.39 However, some clinical and laboratory features that correlate with ADAMTS13 deficiency may be considered as risk factors for acquired TTP (ie, female sex,6,10,40 black ethnicity,6,40,41 HLA-DRB1*11,42 and obesity), whereas HLA-DRB1*04 is protective (Figure 3).6,40 In addition, pathophysiologic conditions increasing plasma VWF levels are established to potentially act as triggering factors of acute TTP episodes (Figure 3).43
Diagnosis
Clinical spectrum of TTP
Today, the historical clinical pentad of fever, thrombocytopenia, microangiopathic hemolytic anemia, neurological symptoms, and renal insufficiency that used to define TTP appears obsolete, as several cohort studies have clearly demonstrated that these 5 symptoms were present in less than 10% of patients with an acute TTP.3,5,7,9,10,44 The almost constant signs of TTP remain severe thrombocytopenia (typically <30 × 109/L) and microangiopathic hemolytic anemia characterized by schistocytes on the blood smear (Figure 2), often associated with corresponding symptoms (ie, skin and mucosal hemorrhage, weakness, and dyspnea). Symptoms related to organ ischemia/infarction mostly concern the brain (∼60% of patients have neurologic symptoms at presentation, with a broad range from headache and confusion to stroke, coma, and seizures).3,5-10 Heart ischemia is also frequent (∼25% of patients, ranging from isolated electrocardiographic abnormalities to myocardial infarction),45 as well as mesenteric ischemia (∼35%) causing abdominal pain and sometimes diarrhea.10 Renal manifestations consist mainly of an isolated proteinuria/hematuria; acute renal failure is unusual in TTP, with typically a serum creatinine level below 2 mg/dL at presentation. However, acute renal failure may not exclude TTP diagnosis, as some studies involving patients with severe TTP (with confirmed ADAMTS13 activity <10%) have reported 10% to 27% acute kidney injury.46
In addition to these manifestations specifically related to the widespread microthrombi of TTP, some patients may have additional signs related to another clinical condition preexisting or concomitant to TTP occurrence. These “nonidiopathic” TTP represent about 50% of all TTP.3,5-10 The most frequent clinical conditions associated with TTP are bacterial infections and autoimmune diseases (mainly systemic lupus erythematous [SLE], but also the antiphospholipid syndrome, Gougerot-Sjögren syndrome), pregnancy, drugs (mitomycin C, cyclosporine, quinine, clopidogrel, ticlopidine), HIV infection, pancreatitis, cancers, and organ transplantation, with some of them being likely involved in the triggering mechanism of the TTP episode (Table 1). In contrast, ∼50% of TTP have an idiopathic presentation (no associated clinical condition).3,5-10
Main associated clinical contexts identified at the initial acute episode of TTP in reports involving more than 50 patients (both adults and children)
| Series (year) . | USS, % . | Idiopathic, % . | Infection* and HIV, % . | Autoimmune disease, % . | Cancer and/or organ/HSPC transplant, % . | Pregnancy and postpartum, % . | Other, % . | Drugs, % . |
|---|---|---|---|---|---|---|---|---|
| Scully et al (2008)3 [N = 176] | 5 | 77 | <1 and 7 | — | 2 | 5† | 4 | <1 |
| Kremer Hovinga et al (2010), Deford et al (2013)6,8 [N = 60] | 0 | 77 | 7 | 5 | 2 and 2 | 5 | 3 | 0 |
| Lotta et al (2010)4 [N = 136] | 0 | 79 | 0 | 7 | 0 | 9 | 1 | 4 |
| Fujimura et al (2010)5 [N = 326] | 12 | 60 | 0 | 14 | 2 and 0 | 1 | 4 | 7 |
| Jang et al (2011)7 [N = 66] | 0 | 59 | 9 | 6 | 8 and 1 | 6 | 3 | 8 |
| Blombery et al (2016)9 [N = 57] | 0 | 75 | 3 and 0 | 18 | 0 | 2 | 0 | 2 |
| Coppo et al (2016)10 [N = 772] | 3 | 49 | 12 and 3 | 11 | 9 and 4 | 5 | 3 | 1 |
| Series (year) . | USS, % . | Idiopathic, % . | Infection* and HIV, % . | Autoimmune disease, % . | Cancer and/or organ/HSPC transplant, % . | Pregnancy and postpartum, % . | Other, % . | Drugs, % . |
|---|---|---|---|---|---|---|---|---|
| Scully et al (2008)3 [N = 176] | 5 | 77 | <1 and 7 | — | 2 | 5† | 4 | <1 |
| Kremer Hovinga et al (2010), Deford et al (2013)6,8 [N = 60] | 0 | 77 | 7 | 5 | 2 and 2 | 5 | 3 | 0 |
| Lotta et al (2010)4 [N = 136] | 0 | 79 | 0 | 7 | 0 | 9 | 1 | 4 |
| Fujimura et al (2010)5 [N = 326] | 12 | 60 | 0 | 14 | 2 and 0 | 1 | 4 | 7 |
| Jang et al (2011)7 [N = 66] | 0 | 59 | 9 | 6 | 8 and 1 | 6 | 3 | 8 |
| Blombery et al (2016)9 [N = 57] | 0 | 75 | 3 and 0 | 18 | 0 | 2 | 0 | 2 |
| Coppo et al (2016)10 [N = 772] | 3 | 49 | 12 and 3 | 11 | 9 and 4 | 5 | 3 | 1 |
HIV, human immunodeficiency virus; HPSC, hematopoietic stem-cell; N, number of patients.
Specific diagnosis made.
Pregnancy and combined oral contraceptive pill.
Differential diagnosis for TTP
The main differential diagnosis for TTP is hemolytic uremic syndrome (HUS), another TMA linked to either Shiga toxin-producing Escherichia coli or abnormalities of proteins of the alternative complement pathway (atypical HUS, aHUS).47 The other differential diagnosis for TTP are other TMA syndromes (most of them being associated with another disease such as cancer, organ transplantation, sepsis, or pregnancy for preeclampsia and the hemolysis elevated liver enzymes low platelet count syndrome), and also both hematological abnormalities (Evans syndrome or isolated thrombocytopenia or isolated hemolytic anemia) and ischemic manifestations linked to autoimmune diseases (mainly SLE, immune thrombocytopenia with the antiphospholipid syndrome).14 To distinguish TTP from other TMA and/or other diseases is crucial because patients with severe ADAMTS13 deficiency are likely to respond to TPE, whereas those without ADAMTS13 severe deficiency often require treatments other than TPE.14 Obstetric TMA are exceptions to this observation because TPE may be beneficial in some instances of hemolysis elevated liver enzymes low platelet count syndrome.48 To definitely identify TTP out of other diagnosis is also crucial because it has a specific outcome requiring a well-defined follow-up.
Laboratory investigation for TTP
Standard analysis.
In addition to the microangiopathic hemolytic anemia and consumption thrombocytopenia, classical parameters for hemolysis show a high reticulocyte count (>120 × 109/L), an undetectable serum haptoglobin concentration, and an elevated lactate dehydrogenase level, a marker for tissue damage.49 The presence of schistocytes on the blood smear (helmet cells; small, irregular triangular, or crescent-shaped cells; pointed projections; and lack of central pallor) with a confident threshold value of 1% is the morphologic hallmark of the disease. Except in some associated autoimmune contexts (SLE), the erythrocyte Coombs’ test is negative. Standard coagulation parameters are usually normal. Renal testing may show proteinuria, hematuria, and sometimes increased plasma urea and creatinine levels. An increased cardiac troponin level (>0.1 µg/L) is present in up to 60% of cases, the majority of whom have no clinical cardiac involvement.45 Electrocardiogram changes, mainly repolarization disorders, are present in 10% of cases.49 Point-based TTP prediction scores have been validated to predict an acquired ADAMTS13 deficiency. These scores include platelet count, serum creatinine level, and either detectable antinuclear antibodies50 or d-dimer, reticulocytes, and indirect bilirubin.51
As these standard investigations are not specific for TTP and may be present in the miscellaneous differential diagnosis for TTP, they should be complemented by analysis of ADAMTS13, the unique marker sensitive and specific for TTP.
ADAMTS13 investigation.
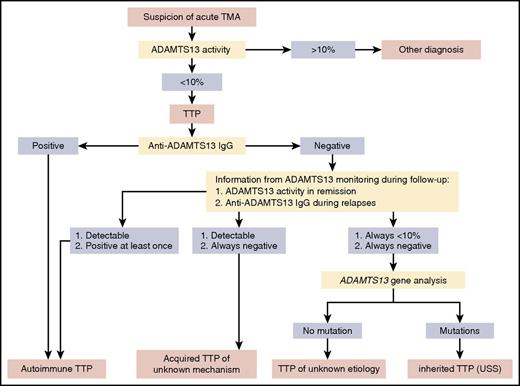
Screening for ADAMTS13 activity is the first test to be performed (Figure 4). If ADAMTS13 activity is less than 10%, TTP diagnosis is confirmed. Several assays are available for ADAMTS13 activity measurement, which in principle consists of degrading a VWF substrate (either full-length VWF19,20,52,53 or small peptide of VWF54-56 ) with ADAMTS13 of the tested citrated plasma or serum. Values are usually expressed as a percentage of the ADAMTS13 activity in normal pooled plasma, defined as 100%, and ideally calibrated against the new World Health Organization international standard ADAMTS13 plasma.57
Flowchart for ADAMTS13 investigation in TTP. ADAMTS13 investigation is mandatory in the management of TMA because it is the unique marker able to definitely establish the diagnosis of TTP. ADAMTS13 activity is always the screening assay to perform. If ADAMTS13 activity is less than 10%, the clinical suspicion of TTP is confirmed. Thus, to document the mechanism for ADAMTS13 severe deficiency, detection of anti-ADAMTS13 IgG is the second-rank assay, whereas ADAMTS13 gene sequencing is a third-rank assay limited to selected indications. Data provided by ADAMTS13 monitoring during follow-up are also important to elucidate the mechanism for ADAMTS13 severe deficiency. In almost all cases, this panel of assays performed during both TTP inaugural episode and follow-up allows us to identify the 3 forms of TTP: acquired autoimmune TTP, acquired TTP of unknown mechanism (apparently not linked to ADAMTS13 autoantibodies), and inherited TTP (USS). In some exceptional cases, however, TTP remains with an unknown etiology.
Flowchart for ADAMTS13 investigation in TTP. ADAMTS13 investigation is mandatory in the management of TMA because it is the unique marker able to definitely establish the diagnosis of TTP. ADAMTS13 activity is always the screening assay to perform. If ADAMTS13 activity is less than 10%, the clinical suspicion of TTP is confirmed. Thus, to document the mechanism for ADAMTS13 severe deficiency, detection of anti-ADAMTS13 IgG is the second-rank assay, whereas ADAMTS13 gene sequencing is a third-rank assay limited to selected indications. Data provided by ADAMTS13 monitoring during follow-up are also important to elucidate the mechanism for ADAMTS13 severe deficiency. In almost all cases, this panel of assays performed during both TTP inaugural episode and follow-up allows us to identify the 3 forms of TTP: acquired autoimmune TTP, acquired TTP of unknown mechanism (apparently not linked to ADAMTS13 autoantibodies), and inherited TTP (USS). In some exceptional cases, however, TTP remains with an unknown etiology.
Functional assays for ADAMTS13.
Reference methods for ADAMTS13 activity remain homemade manual methods requiring substantial skill to provide enough reliability for diagnostic use, especially because of preanalytical and analytical limitations.31,58 Collagen-binding activity and FRETS-VWF73-based assays are adopted reference methods for ADAMTS13 activity measurement,54,59 whereas FRETS-VWF73 is probably superior to collagen-binding activity assay.60 These methods may also be time-consuming, with the shortest one (FRETS-VWF73 assay)54 requiring several labor-intense hours for turnaround of results. As a consequence, these reference methods are limited to expert laboratories (usually 1 or 2 laboratories per country worldwide centralizing ADAMTS13 biology and networking with clinical centers involved in the management of patients with TMA). Implementation of rapid turnaround assay for ADAMTS13 activity, resulting in an accurate diagnosis with a short turnaround time, should be useful to avoid TPE in patients who do not have TTP.61 Inhibitory ADAMTS13 autoantibodies are also detected using functional assays (tested plasma samples are incubated with standard human plasma before residual ADAMTS13 activity measurement).19,20,22
Immunochemical assays for ADAMTS13.
Rapid commercial ELISA assays for ADAMTS13 activity manageable in local laboratories were recently developed, but they do not have the accuracy and the reliability of the reference methods.55,56,62 ADAMTS13 autoantibodies assays, mainly titration of anti-ADAMTS13 IgG using commercial kits,27,36 are relatively simple and are easily performed in a routine laboratory. These assays are secondary, but in the acute setting, when positive, they reinforce the diagnosis of idiopathic TTP.
For all these reasons, reliable results of ADAMTS13 investigation usually cannot be available in an emergency.14 In a large majority of cases, however, the unavailability of ADAMTS13 data in an emergency is not a limitation to initial management. Indeed, after a blood sample was quickly collected from the patient with TMA at presentation for later ADAMTS13 investigation (to avoid any interference with ADAMTS13 supplied by TPE), physicians use only clinical arguments to initiate the first-line treatment in emergency. In other words, urgent therapeutic management is usually decided on the basis of TTP clinical symptoms, and not on the basis of ADAMTS13 results.14,17,63 However, ADAMTS13 investigation remains crucial to definitely confirm TTP diagnosis.14
Focus on some specific TTP cases
Obstetric TTP
Pregnancy-associated TTP mostly occurs during the second half of pregnancy. In the case of USS, TTP occurs as soon as the first pregnancy. In the acquired form of TTP, the first episode may occur during any pregnancy. The rate of fetal loss reported in the literature remains high (∼40%), but studies are mainly retrospective and include a high proportion of women misdiagnosed and lately managed for TTP.36,64 In contrast to other obstetrical TMA (preeclampsia or hemolysis elevated liver enzymes low platelet count syndrome), the fetus extraction does not correct TTP symptoms; as a consequence, provided there are no fetal abnormalities, the pregnancy should not be interrupted, and the patient should be treated using the usual recommendations.64 Among adulthood-onset TTP, obstetric TTP is characterized by a very high frequency of USS (∼33%), with this rate reaching almost 50% when considering only first pregnancies.10,64
Idiopathic TTP and TTP associated with another autoimmune disease
These TTP forms have 2 main common features underlining the strong autoimmune background of TTP: a female predominance (sex ratio ∼2.5 females to 1 male) and a high rate (∼90%) of positive ADAMTS13 autoantibodies during acute TTP events.3,5,7,9,10 In addition, during follow-up, ∼10% of patients with idiopathic TTP develop other autoantibodies either isolated or associated with clinical symptoms of another autoimmune disease (mainly anti-dsDNA and SLE).65
HIV-associated TTP
TTP can typically reveal a HIV infection that needs to be rapidly identified. HIV infection does not alter the usually favorable prognosis of TTP, and response to plasma therapy as well as remission and survival rates are comparable to those of patients with idiopathic TTP. Such patients should therefore benefit from the usual measures in TTP management (eg, intensive and possibly prolonged plasma therapy with TPE), along with highly active antiretroviral therapy.66
Cancer- and organ transplantation-associated TTP
Among all TMA suspicion in patients with cancer, hematopoietic stem cell transplantation, or organ transplantation, those whose ADAMTS13 activity is undetectable at presentation are few. These forms of TTP occur equally in men and women, have an older age of onset, are not the result of an immune-mediated ADAMTS13 deficiency, and portend a worse prognosis when compared with other forms of TTP.10,67 Cancer- and organ transplantation-associated TTP are poorly responsive to TPE. In addition, cancer may be either a known preexisting condition to TTP or discovered concomitantly.
Childhood- and adolescent-onset TTP.
Childhood- and adolescent-onset TTP represent less than 10% of all TTP cases (Figure 1). Because of their rarity, pediatric TTP is often misdiagnosed (∼30% of initial misdiagnosis as HUS, immune thrombocytopenia, Evans syndrome, or malignant hemopathy),12,13 which may worsen prognosis. In contrast to adulthood-onset TTP, the rate of USS appears important in pediatric TTP68,69 : 1 recent study from the French Reference Center for TMA reported, among 68 well-documented pediatric TTP cases enrolled during a 16-year period, 23 USS (33%) and 45 acquired TTP (77%).13 In addition to a distinct mechanism for ADAMTS13 severe deficiency, pediatric USS and pediatric acquired TTP have specific features.70,71
Childhood-onset USS is actually well-described, thanks to registries for genetic rare diseases (eg, http://www.ttpregistry.net). The initial episode of USS occurs typically during infancy (neonatal period) or early childhood (<10 years), and rarely during adolescence.2,72 The severity of the disease (ie, intensity of clinical signs during the acute events and frequency of relapses) is variable and may be related to specific ADAMTS13 mutations73 and to additional genetic or environmental factors. Some heterogeneity of USS symptoms is sometimes reported among siblings whereas the parents, carriers of a single heterozygous ADAMTS13 mutation, are clinically asymptomatic.37
Child-/adolescent-onset acquired TTP is less often reported in the literature.12,13 In the most recent dedicated cohort study describing the inaugural acute phase and the follow-up of 45 patients,13 pediatric acquired TTP is interestingly shown to be quite similar to adulthood-onset acquired TTP: the distribution between idiopathic presentation and nonidiopathic presentation is 55%/45%, the associated clinical conditions of nonidiopathic TTP are mainly infections and autoimmune diseases, and the most common mechanism for ADAMTS13 acquired severe deficiency is ADAMTS13 autoantibodies (80%). Idiopathic TTP is characterized by an adolescence onset (age >10 years), a feminine predominance, and a high frequency of positive ADAMTS13 autoantibodies (96%). In contrast, nonidiopathic TTP is characterized by a younger age (<10 years), a more frequently impaired renal function, and a lower rate of positive ADAMTS13 autoantibodies (65%).13
Prognosis and follow-up
With prompt initiation of TPE, the average survival rate from a first episode of TTP is 80% to 90%.24,74 Older age; a very high lactate dehydrogenase (LDH) level (10 times the upper normal value), reflecting mostly organ damage; and an increased cardiac troponin level on diagnosis (troponin level >0.25 ng/mL) are associated with death and treatment refractoriness.45,75,76
Despite normal physical examination and laboratory parameters,77 a substantial proportion of TTP survivors reported incomplete recovery, with deficits in health-related quality of life.78 Neurocognitive deficits, arterial hypertension, and major depression are reported to be more prevalent than expected in survivors of TTP compared with the healthy population, which may translate into an increased mortality.8 Moreover, patients with TTP are also prone to develop associated connective tissue diseases (mainly SLE and Gougerot-Sjögren syndrome), which require identification early during follow-up.65
Long-term follow-up of patients with TTP, including medical consultation, standard biology, and ADAMTS13 activity monitoring, is also justified by the relapsing risk of this disease. In 40% of cases, patients with autoimmune TTP experience 1 or multiple relapses.6,27,79 Relapses result from severe ADAMTS13 deficiency caused by the persistence or recurrence of anti-ADAMTS13 antibodies.27 So far, ADAMTS13 is the only specific biological marker able to predict TTP relapses during the follow-up in clinical remission.79
Treatment
A complete response to treatment is defined by a platelet count above 150 × 109/L for 2 consecutive days, together with normal or normalizing LDH and clinical recovery. A durable treatment response is lasting at least 30 days after discontinuation of TPE. Recurrent disease within 30 days after reaching treatment response defines an exacerbation, and recurrent disease 30 days or longer after reaching treatment response is a relapse. A refractory disease is defined by no treatment response by day 30 and/or no durable treatment response by day 60.80
Frontline treatment
TTP requires a rapid diagnosis and urgent management, usually in intensive care units, as a medical emergency.
Plasma therapy.
TPE with replacement of plasma remains the cornerstone of current management of TTP (Figure 5).17 According to previous studies,17,81 TPE (usually 1.5× plasma volume exchange for the first procedures, followed by 1.0× patient plasma volume thereafter) should be started as soon as the diagnosis of TTP is established or even suspected. TPE is performed daily until features related to organ involvement (cerebral manifestations, renal failure, increased troponin level, abdominal pain resulting from enteritis or pancreatitis) have resolved, the platelet count has stably recovered, and hemolysis has ceased. Some groups suggested progressively decreasing TPE sessions typically within 3 weeks to prevent severe exacerbations.82 However, this attitude remains debated, particularly as the increasing use of rituximab should better prevent such complications. Various therapeutical plasmas are available: quarantine fresh frozen plasma, plasma virally attenuated with solvent/detergent (OctaplasLG, Octapharma), or a psoralen-derivative (amotosalen) (plasma Intercept, Cerus). All these plasmas are considered equivalent.83 A theoretical superiority of cryosupernatant plasma, which is depleted of high-molecular-weight VWF multimers, has been suggested.84 This was not corroborated; however, in a small randomized trial, the equipotency with plasma has been demonstrated.85 Moreover, this plasma is not available in all countries; therefore, the reported clinical experience is more limited.
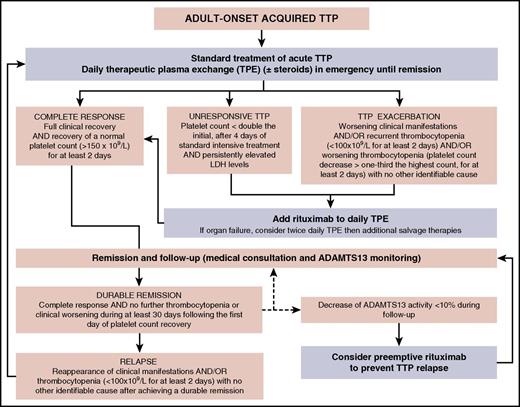
Flowchart for the therapeutic management of adult-onset acquired TTP. The standard treatment of adult-onset acute acquired TTP is daily therapeutic plasma exchange (±steroids) initiated in emergency. This first-line treatment may induce a complete response, but some patients may also be unresponsive, or they may exhibit an exacerbation. In both these latter cases, twice-daily plasma exchange may be prescribed together with rituximab, with this second-line treatment usually leading to complete remission. Follow-up of patients in remission (consisting of medical consultation with standard biology and ADAMTS13 monitoring) may show either a durable remission or relapses requiring standard treatment (with variable outcomes similar to those of the inaugural acute event). In some cases, ADAMTS13 monitoring allows us to identify a decrease of ADAMTS13 activity less than 10%, in the absence of clinical relapse. Considering the high risk for relapse in this situation, it is reasonable to consider a preemptive treatment with rituximab.
Flowchart for the therapeutic management of adult-onset acquired TTP. The standard treatment of adult-onset acute acquired TTP is daily therapeutic plasma exchange (±steroids) initiated in emergency. This first-line treatment may induce a complete response, but some patients may also be unresponsive, or they may exhibit an exacerbation. In both these latter cases, twice-daily plasma exchange may be prescribed together with rituximab, with this second-line treatment usually leading to complete remission. Follow-up of patients in remission (consisting of medical consultation with standard biology and ADAMTS13 monitoring) may show either a durable remission or relapses requiring standard treatment (with variable outcomes similar to those of the inaugural acute event). In some cases, ADAMTS13 monitoring allows us to identify a decrease of ADAMTS13 activity less than 10%, in the absence of clinical relapse. Considering the high risk for relapse in this situation, it is reasonable to consider a preemptive treatment with rituximab.
Steroids.
There is a rationale for the use of steroids in the treatment of acquired TTP, given its autoimmune nature. High-dose methylprednisolone (10 mg/kg/day for 3 days and then 2.5 mg/kg/day) may be more efficacious than standard-dose (1 mg/kg/day) as an adjunctive treatment to TPE in patients with newly diagnosed TTP.86 Taken together, these results indicate steroids might have a place in the management of TTP in association with TPE, although the modality of administration remains debatable. In the absence of evidence-based studies, the empirical use is to systematically associate steroids (1.5 mg/kg/d for 3 weeks) to TPE in the absence of obvious contraindication. However, the level of proof concerning steroid efficacy in the treatment of TTP remains quite low.17,87
Rituximab.
The humanized anti-CD20 monoclonal antibody rituximab was first introduced in patients with a suboptimal response to TTP conventional treatment (ie, disease exacerbation or refractoriness), whereas TPE was usually continued daily.82,88,89 In 4 retrospective studies, 57 patients with TTP were treated with rituximab (in most instances, 375 mg/m2 in 4 weekly doses) after suboptimal response to standard treatment (Tables 2 and 3).90-93 Remission was achieved in 51/57 (89%) cases, typically in less than 4 weeks. Six patients did not respond to treatment, and 3 died. In 2 prospective studies,82,94 involving 47 patients with a refractory or recurrent disease, rituximab 375 mg/m2 administrated within 2 to 3 weeks resulted in remission in 98% of cases within the first month of diagnosis (Tables 2 and 3). No relapse was observed during the first year of follow-up, but there were relapses beyond 1 year. In neither study was rituximab associated with significant adverse effects.
Treatment with rituximab in the acute phase of autoimmune TTP, in reports involving 10 or more patients
| Series (year) . | N . | Age (yr), median (range), mean±SD . | %F . | %R . | Number of rituximab infusion, median (range) . | CR (%) . | Days to CR, median (range), mean ± SD . |
|---|---|---|---|---|---|---|---|
| Scully et al (2007)94 | 25 | 43 (17-67) | 76 | 44 | 4 (2-8) | 100 | 11 |
| Jasti et al (2008)90 | 12 | 43 (19-59) | 83 | 8 | (1-13) | 83 | 18 (14-41) |
| Ling et al (2009)91 | 13 | 42 (23-71) | 69 | 54 | NA | 92 | NA |
| de la Rubia et al (2010)92 | 24 | (24-72) | 71 | 42 | 4 (1-8) | 87.5 | 14 (7-35) |
| Scully et al (2011)89 | 40 | 42 (21-76) | 65 | 15 | 4 (2-8) | 82.5 | 12 |
| Froissart et al (2012)82 | 22 | 36.8 ± 11 | 67 | 14 | 4 | 82 | 12 ± 6.7 |
| Page et al (2016)93 | 16 | 41 (20-79) | 75 | 0 | 4 (1-4) | 100 | 21* (5-76) |
| Series (year) . | N . | Age (yr), median (range), mean±SD . | %F . | %R . | Number of rituximab infusion, median (range) . | CR (%) . | Days to CR, median (range), mean ± SD . |
|---|---|---|---|---|---|---|---|
| Scully et al (2007)94 | 25 | 43 (17-67) | 76 | 44 | 4 (2-8) | 100 | 11 |
| Jasti et al (2008)90 | 12 | 43 (19-59) | 83 | 8 | (1-13) | 83 | 18 (14-41) |
| Ling et al (2009)91 | 13 | 42 (23-71) | 69 | 54 | NA | 92 | NA |
| de la Rubia et al (2010)92 | 24 | (24-72) | 71 | 42 | 4 (1-8) | 87.5 | 14 (7-35) |
| Scully et al (2011)89 | 40 | 42 (21-76) | 65 | 15 | 4 (2-8) | 82.5 | 12 |
| Froissart et al (2012)82 | 22 | 36.8 ± 11 | 67 | 14 | 4 | 82 | 12 ± 6.7 |
| Page et al (2016)93 | 16 | 41 (20-79) | 75 | 0 | 4 (1-4) | 100 | 21* (5-76) |
CR, complete remission (durable treatment response at least 30 d after discontinuation of TPE; F, female; N, number of patients; NA, data not available; R, relapsing patients (recurrent disease ≥ 30 d after reaching treatment response); yr, year.
*Days from first to last TPE.
Clinical relapses and adverse events after treatment with rituximab in the acute phase of autoimmune TTP, in reports involving 10 or more patients
| Series (year) . | Clinical relapse (%) . | Time to relapse, median (range) . | Clinical RFS* (months), median (range) . | Serious adverse events . |
|---|---|---|---|---|
| Scully et al (2007)94 | 0 | — | 10 (1-33 mo) | 1 fatal pneumonia, 1 morbilliform rash |
| Jasti et al (2008)90 | 8 | 23 mo | 48.5 (1-79 mo) | 1 VZV transverse myelitis and encephalitis |
| Ling et al (2009)91 | 0 | — | 24 (13-84 mo) | 0 |
| de la Rubia et al (2010)92 | 12.5 | 29 mo (7-29 mo) | 30 (7.5-64 mo) | 0 |
| Scully et al (2011)89 | 10 | 27 mo (17-31 mo) | ≥12 | 0 |
| Froissart et al (2012)82 | 14 | 24 mo (20-36 mo) | 33 ± 17.4 | 0 |
| Page et al (2016)93 | 12.5* | 2.5 and 9.9 y | — | 0 |
| Series (year) . | Clinical relapse (%) . | Time to relapse, median (range) . | Clinical RFS* (months), median (range) . | Serious adverse events . |
|---|---|---|---|---|
| Scully et al (2007)94 | 0 | — | 10 (1-33 mo) | 1 fatal pneumonia, 1 morbilliform rash |
| Jasti et al (2008)90 | 8 | 23 mo | 48.5 (1-79 mo) | 1 VZV transverse myelitis and encephalitis |
| Ling et al (2009)91 | 0 | — | 24 (13-84 mo) | 0 |
| de la Rubia et al (2010)92 | 12.5 | 29 mo (7-29 mo) | 30 (7.5-64 mo) | 0 |
| Scully et al (2011)89 | 10 | 27 mo (17-31 mo) | ≥12 | 0 |
| Froissart et al (2012)82 | 14 | 24 mo (20-36 mo) | 33 ± 17.4 | 0 |
| Page et al (2016)93 | 12.5* | 2.5 and 9.9 y | — | 0 |
RFS, relapse-free survival; VZV, varicella zoster virus.
*Two relapses.
The indication of rituximab in acquired TTP at the acute phase is still debated. Rituximab was initially associated with daily TPE in patients with a suboptimal response to standard treatment (refractory patients or patients experiencing an exacerbation of the disease). However, the high response rates reported with this therapy provided confidence in its use and prompted investigators to administrate it earlier in the management of the disease. In this regard, the UK group89 reported in 2011 that frontline treatment with rituximab resulted in a shorter hospitalization and fewer relapses that occurred later in comparison with a historical group not treated with rituximab (Tables 2 and 3). Fewer and later relapses were also observed in rituximab-treated patients by the French TMA Reference Center Network82 and the Oklahoma TTP registry.93,95 As a consequence, frontline treatment with rituximab is a reasonable indication, although it may expose patients to overtreatment, as in up to 50% of cases, acquired TTP recovers with standard treatment.96 Further studies should determine whether rituximab allows for improving the poorer outcome of the more severe forms of the disease (ie, old patients with cerebral and/or cardiac involvement).45,76
Other immunomodulators.
Vincristine was used mainly in refractory TTP in the prerituximab era. In a literature review of 56 studies and 105 patients, stable remission was achieved in 73% of patients receiving vincristine as secondary or salvage therapy. Today, however, rituximab is usually preferred in acquired TTP.97 Cyclosporine A has been reported as an effective treatment in refractory TTP98 and as frontline treatment in association with TPE. The clinical response correlates with improvement in ADAMTS13 activity and suppression of anti-ADAMTS13 antibodies.99 However, a recent randomized study showed no significant difference in the exacerbation rate between patients treated in adjunct to TPE with cyclosporine A or with steroids, questioning the role of cyclosporine A.100 Today, however, rituximab is usually preferred in acquired TTP, and use of vincristine and cyclosporine A is reserved for patients refractory to other lines of therapy.
New drugs.
In the forthcoming years, new drugs stemming from a better understanding of TTP pathophysiology should advantageously complete the therapeutic arsenal. These include N-acetylcysteine, which inhibits platelet adherence to endothelial cell-anchored soluble ultralarge VWF multimers by reducing their size,101 bortezomib, a proteasome inhibitor aiming at plasma cell depletion,102 and recombinant ADAMTS13 (BAX930),103,104 the evaluation of which is underway (ClinicalTrials.gov identifier: NCT02216084). Recently, an inhibitor of VWF-glycoprotein 1b interaction (caplacizumab, formerly ALX-0081) was assessed in the TITAN trial, a multicenter, randomized placebo-controlled phase 2 study in patients with acquired TTP.105 With caplacizumab, time to platelet recovery was significantly shorter, and biomarkers reflecting ischemic organ damage tended to normalize more rapidly. Moreover, the incidence of exacerbations was reduced. Bleeding events were mild. Given that caplacizumab does not address the underlying autoimmune pathophysiology, there was a high rate of relapse after stopping caplacizumab 30 days after the last TPE session.105 Caplacizumab is currently further evaluated in the HERCULES trial, a multicenter phase 3 study (ClinicalTrials.gov identifier: NCT02553317).
Management of the more severe forms
Rituximab might not be effective for the treatment of unresponsive TTP during the first 2 weeks, with a reported delay in the onset of its effect that may reach 27 days.82 No consensus has been reached on the best approach to treat refractory, life-threatening TTP.106 If a patient does not respond to standard TPE and prednisone, the current use is to increase the intensity of the treatment sequentially, depending on the clinical course by adding rituximab, followed by twice-daily TPE (1.5 volume plasma per session; Figure 4), pulses of cyclophosphamide, bortezomib, and even splenectomy in the more severe cases, with frequently successful outcomes despite the severity of the disease.107,108
Prevention of relapses
Each relapse exposes the patient to death and to complications related to TPE or to intensive care unit hospitalization.109 Therefore, the prevention of relapses in TTP represents a major goal.
These statements provided a rationale to evaluate the efficacy of rituximab in autoimmune TTP as preemptive therapy for patients in clinical remission, but with persistent severe ADAMTS13 deficiency or in whom ADAMTS13 monitored regularly during follow-up becomes less than 10%.79 In this context, rituximab remarkably reduces the incidence of TTP relapse by diminishing the production of anti-ADAMTS13 antibodies and restoring ADAMTS13 activity, which parallels peripheral B-cell depletion. However, in 30% of these patients, ADAMTS13 recovery is not sustained, and further cycles of rituximab were required to maintain detectable ADAMTS13 activity. Although multiple infusions of rituximab may expose patients to infections or other long-term complications, the reported adverse effects of rituximab reported so far are minimal.79 Splenectomy was also reported to significantly decrease relapses.110,111 A comparison of the efficacy of splenectomy versus rituximab to prevent relapses deserves further studies.
In inherited TTP (USS), plasma infusions are usually sufficient to reverse clinical features. Only some patients with USS may be dependent on regular plasma infusions every 2 to 3 weeks.112 Prophylactic plasma infusions are also required in pregnant women with USS to prevent relapses, which is deleterious for both the mother and the fetus. More generally, plasma therapy should be intensified to cover the most frequent triggering factors of TTP acute episode in pediatric patients with USS, such as infections, surgical procedures, and vaccinations.113 Human recombinant ADAMTS13 (rADAMTS13) could be a future alternative replacement therapy for inherited TTP. Animal models using human rADAMTS13 showed promising results.104 In addition, a phase 1 international multicenter study aimed to assess the safety and pharmacokinetics of rADAMTS13 (BAX930) has been initiated in patients with inherited TTP (ClinicalTrials.gov identifier: NCT02216084).
Conclusion
To further improve TTP prognosis, innovative therapeutics will certainly be helpful in the coming years. However, the diagnosis of TTP in an emergency setting is still challenged by the rarity of the disease, which may lead to a delay in management that can affect the prognosis. In this regard, various measures including educational programs for generalists, emergency department physicians, and all other specialists possibly involved in the management of TTP are progressively being developed in a growing number of countries.
Acknowledgments
The authors acknowledge all the members of the French Reference Center for Thrombotic MicroAngiopathies, and especially Sandrine Malot, Sandrine Thouzeau-Benghezal, Sylvaine Savigny, Sophie Capdenat, and Isabelle Turquois for expert technical assistance.
Authorship
Contribution: B.S.J., P.C., and A.V. wrote the manuscript; B.S.J. created the figures, which were critically reviewed by PC and AV.
Conflict-of-interest disclosure: P.C. is a member of the advisory board for Alexion, Octapharma, and Ablynx. A.V. is a member of the French advisory board for Ablynx. The remaining authors declare no competing financial interests.
Correspondence: Agnès Veyradier, Service d’Hématologie Biologique, Hôpital Lariboisière, Assistance Publique–Hôpitaux de Paris, 2, rue Ambroise Paré, 75010 Paris, France; e-mail: agnes.veyradier@aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal