In this issue of Blood, Zaimoku et al demonstrate that the functional loss of the HLA-B4002 allele is common in aplastic anemia (AA) patients, suggesting that this allele plays a major role in the immune attack underlying the pathophysiology of this disease.1
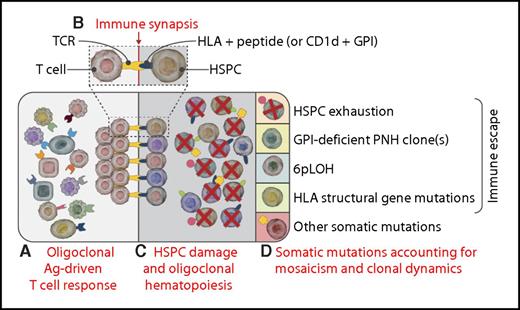
Somatic mutations, hematopoietic mosaicism, and clonal dynamics in immune-mediated AA. (A) From the polyclonal T-cell repertoire, some clonal T cells specific for some antigen expressed on HSPCs (see “Immune synapsis”) may expand, leading to an oligoclonal antigen-driven T-cell response. (B) The immune synapsis: T cells may recognize through their T-cell receptor–specific antigens, presented on (some) HSPCs within either HLA alleles (peptidic epitopes) or HLA-like molecules (for lipidic epitopes; this is the case as with the GPI anchor presented within CD1d).6,7 (C) Pathogenic T-cell clones may exert T-cell–mediated cytotoxicity over many HSPCs (via the immune synapsis depicted in the inset), eventually leading to oligoclonal hematopoiesis.2,4 (D) Different somatic mutations may stochastically occur within individual HSPCs; because of the underlying HSPC oligoclonality, any neutral mutation carried by surviving HSPCs becomes evident (Darwinian selection).9 Individual mutations leading to specific functional phenotypes shape the subsequent hematopoietic mosaicism and clonal dynamics through different mechanisms, including immune escape, HSPC fitness, or proliferative advantage. In the absence of somatic mutations, HSPCs may undergo exhaustion (first quadrant). Expansion of clones escaping the immune response may occur through different mechanisms, such as GPI-deficient cells (PNH, second quadrant)6 or functional loss of HLA due to 6pLOH (third quadrant) or to other structural HLA gene mutations (ie, B4002− cells).1 Other somatic mutations may contribute to clonal dominance through distinct specific mechanisms (fifth quadrant)10 : true malignant transformation for splicing genes, survival/growth advantage, or increased HSPC fitness for epigenetic mutations; unknown (possibly immune escape?) for BCOR-BCORL1 mutations. Ag, antigen; TCR, T-cell receptor. Professional illustration by Somersault18:24.
Somatic mutations, hematopoietic mosaicism, and clonal dynamics in immune-mediated AA. (A) From the polyclonal T-cell repertoire, some clonal T cells specific for some antigen expressed on HSPCs (see “Immune synapsis”) may expand, leading to an oligoclonal antigen-driven T-cell response. (B) The immune synapsis: T cells may recognize through their T-cell receptor–specific antigens, presented on (some) HSPCs within either HLA alleles (peptidic epitopes) or HLA-like molecules (for lipidic epitopes; this is the case as with the GPI anchor presented within CD1d).6,7 (C) Pathogenic T-cell clones may exert T-cell–mediated cytotoxicity over many HSPCs (via the immune synapsis depicted in the inset), eventually leading to oligoclonal hematopoiesis.2,4 (D) Different somatic mutations may stochastically occur within individual HSPCs; because of the underlying HSPC oligoclonality, any neutral mutation carried by surviving HSPCs becomes evident (Darwinian selection).9 Individual mutations leading to specific functional phenotypes shape the subsequent hematopoietic mosaicism and clonal dynamics through different mechanisms, including immune escape, HSPC fitness, or proliferative advantage. In the absence of somatic mutations, HSPCs may undergo exhaustion (first quadrant). Expansion of clones escaping the immune response may occur through different mechanisms, such as GPI-deficient cells (PNH, second quadrant)6 or functional loss of HLA due to 6pLOH (third quadrant) or to other structural HLA gene mutations (ie, B4002− cells).1 Other somatic mutations may contribute to clonal dominance through distinct specific mechanisms (fifth quadrant)10 : true malignant transformation for splicing genes, survival/growth advantage, or increased HSPC fitness for epigenetic mutations; unknown (possibly immune escape?) for BCOR-BCORL1 mutations. Ag, antigen; TCR, T-cell receptor. Professional illustration by Somersault18:24.
The immune-mediated pathophysiology of AA is substantiated by the excellent clinical response to immunosuppressive treatment, and supported by a plethora of experimental studies.2 Most studies suggest that AA is attributable to a T-cell–mediated immune attack targeting hematopoietic stem/progenitor cells (HSPCs). Indeed, different groups have documented in AA the presence of an oligoclonal T-cell response in vivo, with cytotoxic activity of these T-cell clones on autologous HSPCs in vitro.3,4 However, although T-cell repertoire oligoclonality suggests the presence of an antigen-driven T-cell response, the identification of putative autoantigen(s) triggering such immune response remains elusive. Different HLA alleles were found associated with AA, including DRB1*1501, DRB1*1502, B*5201, and B*4002.5 Furthermore, neutral copy-number loss of heterozygosity of the short arm of the chromosome 6 (6pLOH) emerged as a relatively common phenomenon in AA,5 suggesting the hypothesis that it may represent a mechanism of immune escape for HSPCs.
In this work, Zaimoku et al confirm that HLA-B*4002 is among the HLA alleles most frequently carried by AA patients, and that 6pLOH is particularly common in these HLA-B*4002 AA patients.5 Using an ultrasensitive flow cytometry assay exploiting a new anti-HLA-B4002 monoclonal antibody, Zaimoku et al demonstrate that HLA-B4002− granulocytes can be found not only in all HLA-B*4002 patients with a 6pLOH, but also in the majority of patients without 6pLOH. Indeed, deep sequencing of HLA-B*4002 in sorted HLA-B4002− granulocytes isolated from these AA patients without 6pLOH documents that the loss of HLA-B4002 was because of somatic mutations in the HLA-B*4002 gene, leading to the specific phenotype of HLA-B4002−A+granulocytes, which cannot be defined as 6pLOH. These HLA-B4002−A+ granulocytes were detected also in AA patients with 6pLOH, leading to the conclusion that HLA-B4002− granulocytes in these patients are a mosaic of cells truly carrying 6pLOH (HLA-B4002−A−) and cells lacking HLA-B4002 because of other structural gene mutations. Indeed, the authors were able to identify different HLA-B*4002 somatic mutations leading to a loss-of-function phenotype. In addition, in the same patients a few missense mutations were found in phenotypically normal (HLA-B4002+) granulocytes. All these observations suggest that these HLA-B4002− cells tend to expand as a result of continuous immune pressure from which they are spared.
The concept of possible immune escape in the context of AA is not a novel concept in bone marrow failure, since it was first introduced by Rotoli and Luzzatto to explain the pathophysiology of clonal expansion of glycosylphosphatidylinositol (GPI)–deficient cells in paroxysmal nocturnal hemoglobinuria (PNH).6 Autoreactive T cells would target normal HSPCs via some GPI-linked protein or via the GPI anchor itself, eventually sparing PNH (GPI-deficient) HSPCs.6 CD1d-restricted, GPI-specific T cells were found with a higher frequency in PNH patients, eventually suggesting that an immune attack targeting the nonpeptidic GPI anchor could account for the expansion of PNH hematopoiesis.7 More recently, the same CD1d-restricted, GPI-specific T cells have been found increased in AA patients, suggesting that the GPI anchor itself may serve as the target antigen even in the autoimmune process underlying AA.8 With their new study, Zaimoku et al show that immune escape in AA may occur also as a result of HLA loss, eventually providing evidence that HLA-restricted antigen(s) may play a role in AA pathophysiology. These novel findings only appear to contradict their previous data,8 because in an autoimmune process like AA, the oligoclonal T-cell response may target different antigens, eventually shaping the clonal dynamic of residual hematopoiesis. In this context, HLA-B4002 seems to have a major role in the presentation of some typical, still unknown peptidic antigens, while CD1d does the same for the glycolipidic GPI anchor. This noncasual role of HLA-B4002 is also supported by the observation that in presence of effective immunosuppressive therapy the aberrant HLA-B4002− cells may reduce as a result of dilution from a restored, phenotypically normal, polyclonal hematopoiesis.1 However, because Zaimoku et al did not perform the same deep investigation on other HLA alleles, it is not clear whether this propensity to somatic mutations and subsequent functional immune selection is specific of HLA-B*4002, or rather it is a broader phenomenon eventually pertaining to any HLA allele.
Recent studies exploiting next-generation sequencing have documented the presence of mutations within different genes frequently involved in myeloid malignancies.9 The work by Zaimoku et al demonstrated a surprisingly high rate of mutations in the HLA-B*4002 gene, supporting an underlying autoimmune attack rather than indicating a propensity to progress toward myeloid malignancies. Unfortunately, this study could not formally investigate the mutation rate in AA HSPCs nor any possible hotspot mutation within the HLA locus. However, the finding described in this article may lead to some speculations (see figure): (1) somatic mutations are frequently detectable in AA, possibly as a result of Darwinian selection (ie, most mutations are neutral, and they emerge simply because of oligoclonal hematopoiesis)9 ; (2) some somatic mutations may eventually lead to clonal expansion because of an immune privilege (ie, the so-called immune escape, which is well established for PNH cells6 and now also for HLA-B4002 cells1 ; it might be hypothesized also for BCOR/BCOR-L mutations); and (3) the causal role of other somatic mutations in terms of possible malignant transformation requires further demonstration (ie, mutations in genes responsible of epigenetic regulation may simply affect clonal dominance, whereas mutations in splicing genes are more likely to confer a malignant phenotype).10
In conclusion, the data from Zaimoku et al spotlights the role of HLA-B4002 in the autoimmune pathophysiology of AA. This allele is involved in the recognition and subsequent damage of normal HSPCs through the presentation of the causative autoantigen and the formation of the immune synapsis required for T-cell–mediated cytotoxicity. Although further studies are needed to identify the candidate antigen(s) causing AA, current data confirm the immune pathophysiology of AA and allow a better understanding of somatic mutations, hematopoietic mosaicism, and clonal dynamics in the context of immune-mediated bone marrow failure syndromes.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal