Abstract
Despite the introduction of direct oral anticoagulants (DOACs), the search for more effective and safer antithrombotic strategies continues. Better understanding of the pathogenesis of thrombosis has fostered 2 new approaches to achieving this goal. First, evidence that thrombin may be as important as platelets to thrombosis at sites of arterial injury and that platelets contribute to venous thrombosis has prompted trials comparing anticoagulants with aspirin for secondary prevention in arterial thrombosis and aspirin with anticoagulants for primary and secondary prevention of venous thrombosis. These studies will help identify novel treatment strategies. Second, emerging data that naturally occurring polyphosphates activate the contact system and that this system is critical for thrombus stabilization and growth have identified factor XII (FXII) and FXI as targets for new anticoagulants that may be even safer than the DOACs. Studies are needed to determine whether FXI or FXII is the better target and to compare the efficacy and safety of these new strategies with current standards of care for the prevention or treatment of thrombosis. Focusing on these advances, this article outlines how treatment strategies for thrombosis are evolving and describes the rationale and approaches to targeting FXII and FXI. These emerging anticoagulant strategies should address unmet needs and reduce the systemic underuse of anticoagulation because of the fear of bleeding.
Introduction
Thrombosis is a major cause of death and disability. Occurring in veins or arteries, thrombosis is the pathology underlying venous thromboembolism (VTE), which includes deep-vein thrombosis (DVT) and pulmonary embolism (PE), as well as most cases of acute coronary syndrome (ACS) and stroke. Together, these conditions are responsible for 1 in 4 deaths worldwide.1 Therefore, because of the burden of disease, the search for more effective and safer antithrombotic drugs continues.
Thrombi are composed of aggregated platelets, fibrin, and trapped cells, but the proportion of these components differs between arterial and venous thrombi.2,3 Platelets predominate in arterial thrombi, which usually form under high-shear conditions at sites of atherosclerotic plaque disruption. In contrast, fibrin is the major component of venous thrombi, which form under low-shear conditions. Traditionally, these distinguishing features have influenced the choice of antithrombotic therapy.4 Thus, antiplatelet drugs have been the mainstay for the prevention and treatment of arterial thrombosis, whereas anticoagulants have been the foundation for management of VTE. However, treatment paradigms are evolving because of mounting evidence of overlap in the pathogenesis of arterial and venous thrombosis and with identification of the role of the contact system in thrombosis. Thus, evidence that thrombin contributes to recurrent ischemia at sites of arterial injury has prompted studies of anticoagulants for management of arterial thrombosis, whereas the observation that platelets contribute to venous thrombosis has prompted studies of aspirin for primary and secondary VTE prevention.5,6 Likewise, the finding that the contact system is important for thrombus stabilization and growth has identified factor XII (FXII) and FXI as targets for new anticoagulants.7 Focusing on these advances, this article highlights the role of the contact system in the pathogenesis of thrombosis, outlines how treatment strategies for thrombosis are evolving, describes the approaches to targeting FXII and FXI, discusses the relative merits of FXII and FXI as targets, and identifies the unmet medical needs these new agents may address.
Pathogenesis of thrombosis
Role of platelets in arterial and venous thrombosis
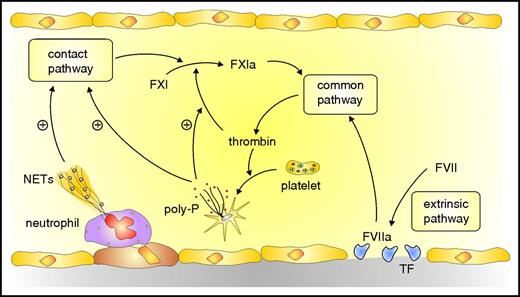
Most arterial thrombi are superimposed on disrupted atherosclerotic plaques. Platelets home to the site of injury and adhere to exposed collagen and von Willebrand factor. Although less abundant in venous thrombi, platelets contribute to their initiation and propagation. Thus, venous thrombosis is attenuated in mice rendered thrombocytopenic,8 in those deficient in von Willebrand factor,9 or in those treated with antiplatelet agents.10 With venous flow restriction, endothelial cells become activated and attract platelets and neutrophils to form heterotypic aggregates. Activated neutrophils release neutrophil extracellular traps (NETs), lattices composed of DNA and histones, to which additional platelets adhere (Figure 1).11 Activated platelets catalyze coagulation via 2 mechanisms. First, clotting factors assemble on their surface to generate thrombin. Second, release of inorganic polyphosphates from platelet dense granules triggers activation of the contact system, which amplifies thrombin generation.12
Overview of coagulation system. Coagulation is initiated by the extrinsic pathway when tissue factor (TF) exposed at sites of vascular injury binds and activates FVII. The activated FVII (FVIIa)–TF complex activates FX in the common pathway to generate prothrombinase, which generates thrombin. Additional activation of coagulation occurs when thrombin-activated platelets release polyphosphate (poly-P) and activated neutrophils extrude DNA and RNA to form NETs. NETs and poly-P activate the contact pathway, which yields FXIa and leads to additional thrombin generation via the common pathway. Poly-P amplifies this pathway by promoting thrombin-mediated activation of FXI.
Overview of coagulation system. Coagulation is initiated by the extrinsic pathway when tissue factor (TF) exposed at sites of vascular injury binds and activates FVII. The activated FVII (FVIIa)–TF complex activates FX in the common pathway to generate prothrombinase, which generates thrombin. Additional activation of coagulation occurs when thrombin-activated platelets release polyphosphate (poly-P) and activated neutrophils extrude DNA and RNA to form NETs. NETs and poly-P activate the contact pathway, which yields FXIa and leads to additional thrombin generation via the common pathway. Poly-P amplifies this pathway by promoting thrombin-mediated activation of FXI.
Tissue factor initiates thrombosis
TF triggers coagulation via the extrinsic pathway by binding and activating FVII. The TF–activated FVII complex, so-called extrinsic tenase, then activates FX and FIX. FIXa binds to FVIIIa on the surface of activated platelets to form intrinsic tenase. Because intrinsic tenase is a better activator of FX than extrinsic tenase, activation of FVIII and FIX is a critical amplification step.13 Therefore, TF initiates thrombin generation via extrinsic tenase and amplifies it via intrinsic tenase.
In most cases of arterial thrombosis, it is TF exposed at sites of atherosclerotic plaque disruption that initiates coagulation. With venous thrombosis, the vein wall is usually intact, but the endothelial cells lining the vein are activated in response to reduced flow or inflammatory mediators. Activated endothelial cells express P-selectin and other adhesion molecules on their surface, which tether TF-expressing leukocytes and microparticles and initiate coagulation. Therefore, TF can trigger arterial and venous thrombosis.
Role of thrombin in thrombosis
Thrombin is the final effector in coagulation because it converts soluble fibrinogen to fibrin, activates platelets, and amplifies its own generation by activating FV and FVIII, key cofactors in coagulation. Thrombin also feeds back to activate FXI, which then activates FIX and augments the formation of intrinsic tenase.14 Therefore, thrombin drives arterial and venous thrombosis.
In patients with ACS, increased thrombin generation persists for 6 to 12 months after the index event.15 As a consequence, vitamin K antagonists (VKAs), such as warfarin, are effective for secondary prevention in patients with acute myocardial infarction, and the addition of low-dose rivaroxaban on top of dual-antiplatelet therapy prevents recurrent ischemia and coronary stent thrombosis in patients with ACS.16
The role of the contact system in thrombosis
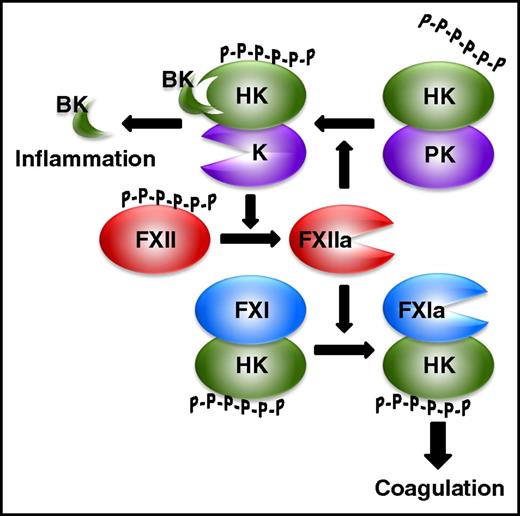
Although it is dispensable for hemostasis, the contact system is essential for thrombus stabilization and growth because thrombi formed at sites of arterial or venous injury in mice deficient in FXII or FXI are small and prone to embolization.17,18 The contact system is composed of 2 zymogens, FXII and prekallikrein, and a cofactor, high-molecular-weight kininogen (HK) (Figure 2).19,20 The system is initiated when FXII binds polyanionic compounds, an interaction that triggers autocatalytic FXII activation. FXIIa converts prekallikrein to kallikrein in a reaction promoted by HK, which binds the polyanion and prekallikrein and positions them in proximity to FXII. Kallikrein activates FXII in a reciprocal manner to generate additional FXIIa, which then activates FXI in a reaction also promoted by HK. FXIa then activates FIX, which incorporates into intrinsic tenase and triggers FX activation and thrombin generation. In addition to activation by FXIIa, FXI can be back-activated by thrombin (Figure 1). Therefore, because the pathways for its activation are bidirectional, FXI is important for maximizing thrombin generation, thereby revealing an important role for the contact system.
The contact system. Polyphosphates (P-P-P-P-P-P) bind FXII and promote its autoactivation to FXIIa. HK binds polyphosphates and promotes FXI activation by FXIIa, and the resultant FXIa then propagates coagulation leading to thrombin generation. FXIIa also activates HK-bound prekallikrein (PK) to kallikrein (K), which activates FXII in a reciprocal manner to promote additional FXIIa generation. Release of bradykinin from HK cleaved by K induces an inflammatory response.
The contact system. Polyphosphates (P-P-P-P-P-P) bind FXII and promote its autoactivation to FXIIa. HK binds polyphosphates and promotes FXI activation by FXIIa, and the resultant FXIa then propagates coagulation leading to thrombin generation. FXIIa also activates HK-bound prekallikrein (PK) to kallikrein (K), which activates FXII in a reciprocal manner to promote additional FXIIa generation. Release of bradykinin from HK cleaved by K induces an inflammatory response.
For decades, the only known activators of the contact system were artificial surfaces, such as kaolin and ellagic acid, and extracorporeal circuits, such as those used for cardiopulmonary bypass or hemodialysis.21,22 Some physiological activators were identified, including heparin, collagen, and denatured proteins, but their involvement in disease was unclear.23,24 This situation changed with the recent demonstration that naturally occurring polyphosphates activate the contact system.11,25 These polyphosphates include DNA and RNA released from injured or dying cells and NETs. Although inorganic polyphosphates released from activated platelets have also been implicated, their role as activators of FXII has been questioned.26 Therefore, generation of these activators at sites of vascular injury provides a stimulus for coagulation distinct from TF. These observations suggest that thrombosis is not a simple imbalance of hemostasis, the physiological process that prevents loss of blood from the vasculature, but rather, the result of alternative stimuli that exploit the hemostatic system.27,28
Evolving treatment paradigms for arterial and venous thrombosis
In parallel with better understanding of the mechanisms of coagulation, treatment modalities have evolved. For years, VKAs were the only available oral anticoagulants. This situation changed with the recent introduction of dabigatran, which inhibits thrombin, and rivaroxaban, apixaban, and edoxaban, which inhibit FXa. These DOACs are more convenient to administer than VKAs because they can be given in fixed doses without routine coagulation monitoring. When compared with VKAs in more than 100 000 patients, the DOACs were at least as effective for stroke prevention in patients with nonvalvular atrial fibrillation and for prevention of recurrence in patients with VTE, but were associated with less bleeding, particularly less intracranial hemorrhage.29 Therefore, many guidelines now give preference to the DOACs over VKAs for stroke prevention in most patients with nonvalvular atrial fibrillation or VTE treatment in patients without active cancer.30,31
With a better understanding of the role of thrombin in arterial thrombosis and the contribution of platelets to venous thrombosis, treatment paradigms are changing. With the greater convenience and better safety profile of the DOACs compared with VKAs, the DOACs are now being investigated for new indications. On the arterial side, these include their comparison with aspirin in patients with embolic stroke of unknown source (ESUS) or coronary or peripheral artery disease, and on the venous side, their comparison with aspirin for primary or secondary thromboprophylaxis (Table 1).
New indications for direct oral anticoagulants
| Indication . | NCT number . | Intervention . | Control . | Duration . | Sample size . | Efficacy outcome . | Safety outcome . |
|---|---|---|---|---|---|---|---|
| Arterial thrombosis | |||||||
| Embolic stroke of unknown source | RE-SPECT ESUS 02239120 | Dabigatran 150 or 110 mg BID | Aspirin | 3 y | 6000 | Recurrent stroke | Major bleeding |
| NAVIGATE ESUS 02313909 | Rivaroxaban 15 mg OD | Aspirin | 3 y | 7000 | Recurrent stroke or systemic embolism | Major bleeding | |
| Coronary or peripheral artery disease | COMPASS 01776424 | Rivaroxaban 5 mg BID or rivaroxaban 2.5 mg BID plus aspirin | Aspirin | 5 y | 27 000 | Major adverse cardiac events | Major bleeding |
| VOYAGER 02504216 | Rivaroxaban 2.5 mg BID on top of aspirin | Placebo on top of aspirin | 2 y | 6500 | Cardiovascular death, myocardial infarction, stroke, acute limb ischemia, or amputation | Major bleeding | |
| Venous thrombosis | |||||||
| Primary prevention | EPCAT II 01720108 | Rivaroxaban 10 mg OD | Aspirin | 10 or 30 d | 3426 | Symptomatic VTE | Major or clinically relevant nonmajor bleeding |
| Secondary prevention | EINSTEIN Choice 02064439 | Rivaroxaban 10 mg OD or rivaroxaban 20 mg OD | Aspirin | 1 y | 3399 | Recurrent VTE | Major bleeding |
| Indication . | NCT number . | Intervention . | Control . | Duration . | Sample size . | Efficacy outcome . | Safety outcome . |
|---|---|---|---|---|---|---|---|
| Arterial thrombosis | |||||||
| Embolic stroke of unknown source | RE-SPECT ESUS 02239120 | Dabigatran 150 or 110 mg BID | Aspirin | 3 y | 6000 | Recurrent stroke | Major bleeding |
| NAVIGATE ESUS 02313909 | Rivaroxaban 15 mg OD | Aspirin | 3 y | 7000 | Recurrent stroke or systemic embolism | Major bleeding | |
| Coronary or peripheral artery disease | COMPASS 01776424 | Rivaroxaban 5 mg BID or rivaroxaban 2.5 mg BID plus aspirin | Aspirin | 5 y | 27 000 | Major adverse cardiac events | Major bleeding |
| VOYAGER 02504216 | Rivaroxaban 2.5 mg BID on top of aspirin | Placebo on top of aspirin | 2 y | 6500 | Cardiovascular death, myocardial infarction, stroke, acute limb ischemia, or amputation | Major bleeding | |
| Venous thrombosis | |||||||
| Primary prevention | EPCAT II 01720108 | Rivaroxaban 10 mg OD | Aspirin | 10 or 30 d | 3426 | Symptomatic VTE | Major or clinically relevant nonmajor bleeding |
| Secondary prevention | EINSTEIN Choice 02064439 | Rivaroxaban 10 mg OD or rivaroxaban 20 mg OD | Aspirin | 1 y | 3399 | Recurrent VTE | Major bleeding |
BID, twice daily; COMPASS, Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease; EINSTEIN Choice, Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism; EPCAT II, Extended Venous Thromboembolism Prophylaxis Comparing Rivaroxaban to Aspirin Following Total Hip and Knee Arthroplasty; NAVIGATE ESUS, Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source; NCT, national clinical trial (clinicaltirals.gov); OD, once daily; RE-SPECT ESUS, Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source; VOYAGER, Efficacy and Safety of Rivaroxaban in Reducing the Risk of Major Thrombotic Vascular Events in Subjects With Symptomatic Peripheral Artery Disease Undergoing Peripheral Revascularization Procedures of the Lower Extremities.
Embolic stroke of unknown source
Previously known as cryptogenic stroke, ESUS represents about 25% of all ischemic strokes. Most of these stokes are embolic in origin, and thrombi in such patients may originate from diverse sources, including the left atrial appendage in those with subclinical atrial fibrillation, deep veins of the leg via paradoxical embolism through right-to-left shunts, and disrupted atherosclerotic plaques in the aortic arch or carotid or cerebral arteries.32 The optimal management of patients with ESUS is uncertain, and at this time, most patients receive aspirin. However, the RE-SPECT ESUS and NAVIGATE ESUS trials are comparing aspirin with dabigatran or with rivaroxaban, respectively, for reducing the risk for recurrent stroke.
Coronary and peripheral artery disease
Patients with coronary or peripheral artery disease are at risk for cardiovascular events.5 Aspirin, the current standard of care, reduces the risk by about 25%. Therefore, there is an unmet need for more effective therapy. The addition of rivaroxaban (2.5 mg twice daily) to dual-antiplatelet therapy in patients with ACS reduced the risk for cardiovascular death, myocardial infarction, and stroke.16 Capitalizing on these findings, the 3-arm COMPASS trial has randomly assigned 27 400 patients with coronary or peripheral artery disease to aspirin, rivaroxaban at a dose of 5 mg twice daily, or the combination of aspirin plus rivaroxaban at a dose of 2.5 mg twice daily.
Focusing on patients undergoing lower limb revascularization for symptomatic peripheral artery disease, the VOYAGER study is investigating whether compared with placebo on top of usual care, which includes aspirin, rivaroxaban at a dose of 2.5 mg twice daily reduces the risk for cardiovascular death, myocardial infarction, stroke, acute limb ischemia, and amputation. Therefore, the COMPASS and VOYAGER studies will investigate the use of rivaroxaban in patients undergoing a revascularization procedure or with stable disease.
Venous thromboembolism
Aspirin is being compared with anticoagulation therapy for primary and secondary VTE prevention in the EPCAT II and EINSTEIN Choice studies, respectively. In EPCAT II, 3426 patients undergoing elective hip or knee arthroplasty who received in-hospital thromboprophylaxis with rivaroxaban (10 mg daily) were randomly assigned at discharge to continued rivaroxaban or to aspirin (81 mg daily) for 30 or 10 days, respectively. The primary efficacy and safety outcomes are symptomatic VTE and the composite of major and clinically relevant nonmajor bleeding, respectively. Therefore, this study compares the efficacy and safety of aspirin and rivaroxaban for extended primary thromboprophylaxis.
When compared with placebo in patients with unprovoked VTE who had received 6 to 12 months of anticoagulation therapy for their index event, aspirin reduced the risk for recurrence by 32% and was associated with a low risk for major bleeding.33 The 3-arm EINSTEIN Choice study randomly assigned 3399 patients with VTE who had completed 6 to 12 months of anticoagulation and had ongoing risk factors for recurrence to rivaroxaban 20 mg once daily, rivaroxaban 10 mg once daily, or aspirin.5 Therefore, this will be the first study to directly compare the efficacy and safety of anticoagulant and antiplatelet strategies for secondary thromboprophylaxis. Although these studies may reveal new indications for the DOACs alone or in combination with aspirin, pursuit of novel targets may be necessary to further reduce the risk of bleeding with anticoagulant therapy. It is this goal that fostered interest in targeting FXII and FXI.
FXII and FXI as targets for new anticoagulants
The ultimate objective of anticoagulation therapy is to attenuate thrombosis without affecting hemostasis. Although the DOACs come closer to this goal than VKAs, there still is room for improvement. Thus, the annual rate of major bleeding with the DOACs in patients with atrial fibrillation is 2% to 3%, whereas the annual rate of intracranial bleeding is 0.3% to 0.5%.34 As a consequence, even with the introduction of the DOACs, more than one-third of patients with atrial fibrillation fail to receive anticoagulant prophylaxis mainly because of the fear of bleeding.35 Therefore, there remains a need for safer anticoagulants.
The DOACs target FXa or thrombin, downstream enzymes in the coagulation cascade. Interest in upstream targets FXII and FXI stems from basic and epidemiological studies that suggest these factors are important in thrombosis.7,20,27 This makes them promising targets for the development of safer anticoagulants because they have little or no role in hemostasis.
Epidemiological data supporting the role for FXI in thrombosis are stronger than those for FXII. Thus, patients with congenital FXI deficiency are protected from VTE and ischemic stroke, patients with higher levels of FXI are at greater risk for VTE and ischemic stroke than those with lower levels, and the levels of FXI correlate with stroke risk in women taking oral contraceptives.36,37 The role of FXI in myocardial infarction is less clear: some studies suggest it is important, and others do not. This discrepancy may reflect differences in study design, or the contribution of FXI to thrombosis in the coronary circulation may be distinct from that in other vascular beds.
Epidemiological evidence for a role of FXII in thrombosis is not strong, but data are limited because FXII deficiency is rare.37 Patients with congenital FXII deficiency do not appear to be at lower risk for VTE or to be protected from ischemic stroke or myocardial infarction. In fact, some studies suggest such patients are at higher risk for thrombotic events. Furthermore, in contrast to FXI, there is no association between increased FXII levels and the risk for VTE. Finally, patients with hereditary angioedema as a consequence of impaired regulation of FXIIa and kallikrein are not prone to thrombosis. Therefore, there is little evidence of a link between FXII and thrombosis in humans.
Despite the epidemiological data in humans, FXI and FXII appear to be equally important drivers of thrombosis in small mammal models. Thus, FXII-deficient mice are protected from ischemic stroke and form smaller thrombi after venous flow restriction.38 Furthermore, mice deficient in FXII or FXI exhibit equally attenuated thrombosis at sites of arterial or venous injury, and the thrombi formed in such mice are unstable under flow conditions and undergo rapid fragmentation.17,18 Likewise, in a rabbit model, FXII knock down with an antisense oligonucleotide (ASO) reduced catheter thrombosis to a similar extent as FXI knock down.39 Therefore, FXII is just as important as FXI for thrombosis in rodents and rabbits.
The results in nonhuman primates are different. Thus, in a baboon arteriovenous shunt model, antibodies against FXI appear to attenuate platelet and fibrin deposition more than those directed against FXII.40,41 In this same model, FXI knockdown with an ASO reduced thrombosis in a concentration-dependent manner once FXI levels were below 50% of normal.42 Therefore, FXI appears to be a more important driver of thrombosis than FXII in nonhuman primate models.
Strategies to target FXII and FXI are illustrated in Table 2 and include ASOs that reduce hepatic synthesis of the clotting proteins,39,42,43 monoclonal antibodies that block activation or activity,40,41,44-46 aptamers,47 and small molecules that block the active site48-50 or induce allosteric modulation.51,52 Each strategy not only differs in terms of mechanism but also has unique pharmacological characteristics (Table 3). Thus, ASOs, antibodies and aptamers require parenteral administration, whereas active site inhibitors have the potential for parenteral or oral delivery. The onset and offset of action also varies. The 3 to 4 weeks of ASO treatment required to lower FXII or FXI levels into the therapeutic range limits their utility for initial treatment of thrombosis or for immediate thromboprophylaxis.39,42,43 The prolonged half-life of FXI-directed antibodies or ASOs could be problematic if there is bleeding with trauma or surgery. Therefore, each strategy has strengths and weaknesses.
Strategies to target factor XII or factor XI
| Strategy . | Mechanism of action . |
|---|---|
| Antisense oligonucleotides | Reduce hepatic synthesis of factor XII or factor XI |
| Aptamers | Bind factor XII or factor XI and block activity |
| Antibodies | Bind factor XII or factor XI and block activation or activity |
| Small molecules | Bind reversibly to active site of factor XIIa or factor XIa and block activity |
| Polyanion antagonists | Neutralize polyphosphates or nucleic acids via ionic interactions, thereby attenuating contact pathway activation |
| Strategy . | Mechanism of action . |
|---|---|
| Antisense oligonucleotides | Reduce hepatic synthesis of factor XII or factor XI |
| Aptamers | Bind factor XII or factor XI and block activity |
| Antibodies | Bind factor XII or factor XI and block activation or activity |
| Small molecules | Bind reversibly to active site of factor XIIa or factor XIa and block activity |
| Polyanion antagonists | Neutralize polyphosphates or nucleic acids via ionic interactions, thereby attenuating contact pathway activation |
Pharmacological features of factor XII– or factor XI–directed strategies
| Feature . | Antisense oligonucleotides . | Antibodies . | Aptamers . | Small molecules . |
|---|---|---|---|---|
| Delivery | Parenteral | Parenteral | Parenteral | Parenteral or oral |
| Specific | Yes | Yes | Yes | Yes |
| Onset of action | Delayed | Immediate | Immediate | Immediate |
| Offset of action | Delayed | Delayed | Rapid | Rapid |
| Renal clearance | No | No | No | Variable |
| Hepatic metabolism | No | No | No | Variable |
| Potential clinical indications | Chronic | Acute or chronic | Acute or chronic | Acute or chronic |
| Feature . | Antisense oligonucleotides . | Antibodies . | Aptamers . | Small molecules . |
|---|---|---|---|---|
| Delivery | Parenteral | Parenteral | Parenteral | Parenteral or oral |
| Specific | Yes | Yes | Yes | Yes |
| Onset of action | Delayed | Immediate | Immediate | Immediate |
| Offset of action | Delayed | Delayed | Rapid | Rapid |
| Renal clearance | No | No | No | Variable |
| Hepatic metabolism | No | No | No | Variable |
| Potential clinical indications | Chronic | Acute or chronic | Acute or chronic | Acute or chronic |
An alternative approach to attenuating contact pathway activation is to nullify the stimulators (Table 3). Thus, agents that neutralize nucleic acids or polyphosphate have been shown to attenuate coagulation and reduce thrombosis in mouse models.53-55 Therefore, there are numerous potential strategies to target the contact pathway to reduce thrombosis.
FXII or FXI: which is the better target?
The advantage of FXII as a target is safety: Because FXII has no role in hemostasis, strategies targeting it will not induce bleeding (Table 4). In contrast, strategies targeting FXI may be associated with bleeding, particularly mucosal bleeding, which occurs in patients with severe FXI deficiency.56 A potential limitation of FXII as a target is that its role in thrombosis is less certain than that of FXI, based on epidemiological data.57-63 In addition, targeting FXII may be of limited benefit when thrombosis is initiated by TF because thrombin generated via extrinsic tenase has the potential to activate FXI, thereby bypassing FXII inhibition.14 Therefore, despite the potential for mild bleeding, FXI may be a better target than FXII for most indications.
Relative advantages and disadvantages of factor XII or factor XI as targets for new anticoagulants
| . | Factor XII . | Factor XI . |
|---|---|---|
| Epidemiological data | Weak | Stronger |
| Risk for bleeding | None | Low |
| Level of evidence for role in thrombosis | Preclinical | Phase 2 |
| Potential for bypassing inhibition | Thrombin-mediated activation of factor XI could bypass factor XII inhibition | None |
| Potential for off target effects | May modulate inflammation by inhibiting bradykinin generation | Unlikely |
| . | Factor XII . | Factor XI . |
|---|---|---|
| Epidemiological data | Weak | Stronger |
| Risk for bleeding | None | Low |
| Level of evidence for role in thrombosis | Preclinical | Phase 2 |
| Potential for bypassing inhibition | Thrombin-mediated activation of factor XI could bypass factor XII inhibition | None |
| Potential for off target effects | May modulate inflammation by inhibiting bradykinin generation | Unlikely |
An exception may be clotting on blood-contacting medical devices or extracorporeal circuits because thrombosis on artificial surfaces is triggered by FXII activation.22 Thus, catheters coated with corn trypsin inhibitor, a potent and specific inhibitor of FXIIa, remain patent longer than uncoated catheters when inserted in the jugular veins of rabbits.64 Likewise, FXII knockdown prolonged the time to occlusion of such catheters by more than 2-fold,39 and a FXIIa-directed antibody was as effective as heparin at preventing clotting in an extracorporeal membrane oxygenation circuit in rabbits, but produced less bleeding.46 Therefore, these studies suggest that contact activation of FXII initiates clotting on artificial surfaces.
Although FXII triggers clotting on artificial surfaces, FXI also is important. FXI knockdown was as effective as FXII knockdown at preventing catheter occlusion in rabbits.39 Furthermore, although FXII depletion reduced thrombin generation induced by components of mechanical heart valves to background levels, FXI depletion abolished it.65 Therefore, strategies targeting FXI may be as or more effective than those targeting FXII for prevention of clotting on artificial surfaces.
The only strategy to be tested in humans is the FXI-directed ASO IONIS-416858, which is given subcutaneously. IONIS-416858 reduced FXI antigen and activity levels in a concentration-dependent manner in healthy volunteers.66 In a phase 2 study, 300 patients undergoing elective knee arthroplasty were randomly assigned to receive IONIS-416858 at doses of 200 or 300 mg starting 35 days before surgery or to enoxaparin at a dose of 40 mg once daily, starting after surgery. Both treatments were continued for at least 10 days, at which point patients underwent bilateral venography.67 The primary efficacy outcome was VTE, which included the composite of asymptomatic DVT, symptomatic DVT or PE, and VTE-related mortality; the principal safety outcome was bleeding. At the time of surgery, mean FXI levels were reduced to 38% and 28% of baseline values in the groups receiving the 200- and 300-mg IONIS-416858 doses, respectively.67 The primary efficacy outcome occurred in 36 (27%) of 134 patients and in 3 (4%) of 71 patients who received the 200- and 300-mg doses of IONIS-416858, respectively, as compared with 21 (30%) of 69 patients who received enoxaparin. The 200-mg IONIS-416858 regimen was noninferior and the 300-mg ASO regimen was superior to enoxaparin (P < .001). The rates of the composite of major or clinically relevant nonmajor bleeding were 3% in both IONIS-416858 groups and 8% in the enoxaparin groups. Therefore, lowering FXI levels reduces the risk for postoperative VTE to a greater extent than enoxaparin, without increasing the risk for bleeding.
The findings of this study change our thinking about the pathogenesis of postoperative venous thrombosis. There is no question that thrombin generation is increased after major orthopedic surgery as a result of TF exposure at the surgical site. There are 2 potential explanations for the reduced risk for VTE with FXI knockdown. First, TF-induced thrombin generation may amplify coagulation by feedback activation of FXI. Second, surgery may trigger the release of DNA and RNA from damaged cells and polyphosphate from activated platelets that directly activate FXII. Knockdown of FXI prevents propagation of coagulation by either pathway, whereas strategies that target FXII only block contact activation. We now need to apply this information to identify potential clinical indications for FXI- or FXII-directed strategies.
Potential indications for FXII- or FXI-directed therapies
Additional studies are needed to confirm the results of FXI-directed strategies for VTE prevention and to identify other indications for FXI- or FXII-directed strategies (Table 5). An unanswered question is whether inhibition of FXII or FXI is sufficient for treatment of established venous or arterial thrombosis when used as sole therapy. Until this question is answered, it may be better to focus on prevention. Because of their slow onset of action, FXII- or FXI-directed ASOs are best suited for chronic indications. These might include secondary prevention in patients with unprovoked VTE, prevention of cardiovascular events in patients with chronic kidney disease, and stroke prevention in patients with atrial fibrillation at high risk of bleeding, such as those with end-stage renal disease who are receiving hemodialysis. Unprovoked VTE is a potential indication because these patients have a risk for recurrent thrombosis of about 10% at 1 year and 30% at 5 years if anticoagulant therapy is stopped.68 For this reason, many of them are maintained on indefinite anticoagulant therapy, which carries a risk of bleeding even with the DOACs.69 FXII- or FXI-directed strategies may be safer, and adherence may be better with once- or twice-monthly injections of ASOs or antibodies than with oral medications that must be taken one or twice daily (Table 5). These possibilities need to be tested.
Potential Indications for factor XII– or factor XI–directed strategies
| Indication . | Rationale . |
|---|---|
| Primary VTE prophylaxis | Long-acting strategies such as antisense oligonucleotides or antibodies permit simple and safe single-dose regimens for extended thromboprophylaxis in medically ill patients or after major orthopedic surgery |
| Secondary VTE prophylaxis | May be safer than current therapies for secondary prevention in patients with unprovoked or cancer-associated venous thromboembolism |
| Prevention of recurrent ischemia after ACS | May provide a safer anticoagulant platform on top of single- or dual-antiplatelet therapy |
| End-stage renal disease | May be safe and effective for reducing cardiovascular death, myocardial infarction, and stroke in patients receiving hemodialysis |
| High-risk atrial fibrillation patients | May be safer than current therapies for stroke prevention in patients with atrial fibrillation at high risk of bleeding such as those with a history of major bleeding or with end-stage renal disease |
| Medical devices | May be more effective and safer than current therapies to prevent clotting on mechanical heart valves, left ventricular assist devices, small caliber grafts, or central venous catheters |
| Extracorporeal circuits | May be more effective and safer than heparin to prevent clotting on extracorporeal membrane oxygenator or cardiopulmonary bypass circuits |
| Indication . | Rationale . |
|---|---|
| Primary VTE prophylaxis | Long-acting strategies such as antisense oligonucleotides or antibodies permit simple and safe single-dose regimens for extended thromboprophylaxis in medically ill patients or after major orthopedic surgery |
| Secondary VTE prophylaxis | May be safer than current therapies for secondary prevention in patients with unprovoked or cancer-associated venous thromboembolism |
| Prevention of recurrent ischemia after ACS | May provide a safer anticoagulant platform on top of single- or dual-antiplatelet therapy |
| End-stage renal disease | May be safe and effective for reducing cardiovascular death, myocardial infarction, and stroke in patients receiving hemodialysis |
| High-risk atrial fibrillation patients | May be safer than current therapies for stroke prevention in patients with atrial fibrillation at high risk of bleeding such as those with a history of major bleeding or with end-stage renal disease |
| Medical devices | May be more effective and safer than current therapies to prevent clotting on mechanical heart valves, left ventricular assist devices, small caliber grafts, or central venous catheters |
| Extracorporeal circuits | May be more effective and safer than heparin to prevent clotting on extracorporeal membrane oxygenator or cardiopulmonary bypass circuits |
Stroke prevention in hemodialysis patients with atrial fibrillation represents an unmet medical need because the DOACs have not been tested in such patients, and because there is uncertainty as to whether the harms of warfarin outweigh its benefits in this setting. FXI is likely to be a better target than FXII for this indication because FXI inhibition will prevent thrombus stabilization and growth, regardless of whether the stimulus for clotting at sites of plaque disruption or in the left atrial appendage is driven by TF or by FXII activation by polyphosphates. Inhibition of FXI may also attenuate clotting on the hemodialysis circuit, thereby obviating the need for heparin and further lowering the risk of bleeding. Even without atrial fibrillation, patients receiving hemodialysis are at risk for cardiovascular events, and such events are responsible for at least 50% of the mortality. Therefore, a FXI-directed strategy may be beneficial to safely prevent such events in hemodialysis patients with or without atrial fibrillation.
FXII- or FXI-directed therapies may be safer than heparin for prevention of clotting on extracorporeal membrane oxygenation circuits, and safer than warfarin for prevention of thromboembolic events in patients with left ventricular assist devices. Dabigatran failed against warfarin in patients with mechanical heart valves,70 a finding that prompted black box warnings against the use of DOACs in such patients. FXI-directed strategies may be very effective in this setting because FXI depletion abolished mechanical valve–induced thrombin generation in vitro.65
FXI-directed strategies may also provide a safer platform than current anticoagulants in patients requiring anticoagulant therapy on top of single- or dual-antiplatelet therapy (Table 5). Thus, even though rivaroxaban reduced the risk for recurrent ischemic events and stent thrombosis when added to dual-antiplatelet therapy in patients with ACS, these beneficial effects came at a cost of increased bleeding, including intracranial bleeding.16 FXI-directed strategies are likely to be safer than rivaroxaban and should not only block contact activation on stents but also prevent FXI-mediated thrombus stabilization and growth.
Conclusions and future directions
Advances in our understanding of the pathogenesis of arterial and venous thrombosis are changing treatment paradigms. Anticoagulants are now being compared with aspirin or combined with aspirin for secondary prevention in patients with stroke or coronary or peripheral artery disease, and aspirin is being compared with anticoagulants for primary or secondary VTE prevention. These studies will help to identify the optimal treatment strategies.
Recent advances have catalyzed examination of targets beyond those involved in the terminal reactions of the coagulation pathway. With evidence that the contact system is important for thrombus stabilization and growth, FXI and FXII have emerged as promising targets for new anticoagulants that are likely to be safer than those that inhibit FXa or thrombin. ASOs, antibodies, and small molecules provide a growing armamentarium of agents to determine whether FXI or FXII is the better target and to compare the efficacy and safety of these new strategies with current standards of care for prevention or treatment of thrombosis. Selection of indications should focus on unmet medical needs, particularly those where current therapies are limited in both efficacy and safety. The clinical potential of FXII- and FXI-directed anticoagulant strategies should be clarified during the next few years.
Acknowledgments
J.I.W. holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J. Fraser Mustard Chair in Cardiovascular Research.
Authorship
Contribution: J.C.F., P.L.G., and J.I.W. wrote the manuscript.
Conflict-of-interest disclosure: P.L.G. has received honoraria from Bayer, Pfizer, Bristol-Myers Squibb, and Leo Pharma. J.I.W. has served as a consultant and has received honoraria from IONIS Pharmaceuticals, Janssen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Merck, and Daiichi Sankyo. J.C.F. declares no competing financial interests.
Correspondence: Jeffrey I. Weitz, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; e-mail: weitzj@taari.ca.