Key Points
Human neutrophils mediate trogocytosis rather than phagocytosis of CD20-antibody–opsonized CLL B cells.
Trogocytosis is induced more effectively by rituximab compared with obinutuzumab.
Abstract
Polymorphonuclear neutrophils (PMNs) have previously been reported to mediate phagocytosis of anti-CD20–opsonized B cells from patients with chronic lymphocytic leukemia (CLL). However, recent data have suggested that PMNs, like macrophages, can also mediate trogocytosis. We have performed experiments to more precisely investigate this point and to discriminate between trogocytosis and phagocytosis. In live-cell time-lapse microscopy experiments, we could not detect any significant phagocytosis by purified PMNs of anti-CD20–opsonized CLL B cells, but could detect only the repeated close contact between effectors and targets, which suggested trogocytosis. Similarly, in flow cytometry assays using CLL B-cell targets labeled with the membrane dye PKH67 and opsonized with rituximab or obinutuzumab, we observed that a mean of 50% and 75% of PMNs had taken a fraction of the dye from CLL B cells at 3 and 20 hours, respectively, with no significant decrease in absolute live or total CLL B-cell numbers, confirming that trogocytosis occurs, rather than phagocytosis. Trogocytosis was accompanied by loss of membrane CD20 from CLL B cells, which was evident with rituximab but not obinutuzumab. We conclude that PMNs mediate mostly trogocytosis rather than phagocytosis of anti-CD20–opsonized CLL B cells, and we discuss the implications of this finding in patients with CLL treated with rituximab or obinutuzumab in vivo.
Introduction
Polymorphonuclear neutrophils (PMNs) are efficient phagocytes for antibody- or complement-opsonized microorganisms. Furthermore, they have been reported to play an important role in the therapeutic activity of anti-CD20 as well as anti-HER2 and melanoma antibodies in several in vivo mouse models.1-3 Thus, it is important to further define PMN function in this context.
PMNs are known to become activated by target cells opsonized by anti-CD20 antibodies, with upregulation of CD11b and downmodulation of CD62L on their surfaces.4 The type II anti-CD20 glycoengineered antibody obinutuzumab (OBZ) was more effective in this activity compared with type I wild-type rituximab (RTX), presumably as a result of the higher binding affinity of the former to CD16 on PMNs. Some ROS production by PMNs has also been reported in the presence of immobilized RTX.5 Previously, we and others also reported that, in vitro, PMNs mediate phagocytosis of chronic lymphocytic leukemia (CLL) B cells in the presence of anti-CD20 antibodies.4,6 Our conclusion was based on direct visualization of PMNs in cytospins and in flow cytometry experiments, using a 3-color immunofluorescence method to distinguish phagocytic PMNs from those that had only bound and not internalized CLL B cells.4,6 Recently, the phenomenon of trogocytosis, mediated in particular by monocytes/macrophages, has been shown to be important in the context of therapeutic antibodies.7,8 Furthermore, some recent reports in the literature have suggested that PMNs also mediate trogocytosis.7,9,10 Our previous flow cytometry assay, however, could not easily discriminate between trogocytosis and phagocytosis, both of which would have been identified as events positive for both the PMN and CLL B-cell membrane markers, but negative for CD19 (given that trogocytosis downmodulates multiple membrane proteins on targets, including CD19).9,11 We therefore set out to further investigate this point, using live-cell time-lapse microscopy, confocal microscopy, and more extensive flow cytometry analyses with different membrane markers. We show that PMNs mediate trogocytosis rather than phagocytosis and that this is accompanied by partial loss of CD20 from the cell surface, but not death of the CLL B-cell targets.
Materials and methods
Antibodies
The following previously described immunoglobulin G1 (IgG1) anti-CD20 antibodies were used: wild-type rituximab (RTX-WT) or glycoengineered (afucosylated) rituximab (RTX-GE), and wild-type obinutuzumab (OBZ-WT) or glycoengineered obinutuzumab (OBZ-GE).4 RTX-WT and OBZ-GE are the antibodies approved for clinical use. All antibodies were provided by Roche Glycart. The negative-control antibody was the anti-EGFR cetuximab (CET), which was obtained from the hospital Papa Giovanni XXIII pharmacy.
Cells
Peripheral blood was drawn from healthy volunteers or patients diagnosed with CLL in EDTA vacutainer tubes. Samples were obtained in accordance with the Declaration of Helsinki of 1975, as revised in 2008. All subjects gave written informed consent for their blood products to be used for research under an institutional review board–approved protocol. PMNs were purified from the peripheral blood of healthy human donors using the MACXpress kit (Miltenyi, Bergish Gladbach, Germany). Purified PMNs were >95% pure by flow cytometry. Peripheral blood mononuclear cells were purified from the peripheral blood of patients with CLL by standard Ficoll-Hypaque centrifugation and stored frozen in 10% dimethyl sulfoxide in liquid nitrogen until use. The samples used in the experiments all contained >90% CD19+CD5+ leukemic target cells. The CD20 expression on CLL B cells was quantified as the number of CD20 molecules per cell using anti-CD20 phycoerythrin and Quantibrite beads (both from BD Biosciences), according to the manufacturer’s instructions.
Live-cell time-lapse microscopy experiments
Purified PMNs were plated with CLL B cells at a 1:2 effector:target (E:T) ratio in X-VIVO 15 medium (Lonza, Basel, Switzerland) in 8-well microslides (ibidi, Planegg, Germany). The slides were placed in a Zeiss Axio observer Z1 microscope chamber (Carl Zeiss Microscopy, Oberkochen, Germany) at 37°C with 5% CO2, and then 10 µg/mL of anti-CD20 or control antibodies (Roche) were added. In control experiments, 4.5-µM Dynabeads coated with T-specific anti-CD3/CD28 antibodies (mouse IgG1 and IgG2a) (Dynal Biotech, Oslo, Norway) were added to the PMN culture instead of opsonized CLL B cells. Differential interference contrast images were taken every 30 seconds for 4 to 6 hours with an AxioCam MRm R31 camera (Carl Zeiss Microscopy), using an LD Plan-Neofluar ×40/0.6 PH2 KORR objective with PlasDIC slider (Carl Zeiss Microscopy). The videos were assembled with AxioVision software (release 4.8; Carl Zeiss Microscopy).
Confocal microscopy
Purified PMN and CLL B cells were labeled with PKH26 and PKH67 dyes (Sigma-Aldrich, St. Louis, MO), respectively, according to the manufacturer’s instructions. The labeled PMNs were then incubated in X-VIVO medium (Lonza) with labeled CLL B cells, at a 1:2 E:T ratio, in the presence or absence of 10 µg/mL of RTX-WT, OBZ-GE, or the control CET antibody, in a 35-mm CellView glass-bottom dish (Greiner Bio-One, Pleidelsheim, Germany). After 3 hours at 37°C with 5% CO2, the slides were washed in phosphate-buffered saline, fixed for 15 minutes in 2% paraformaldehyde, stained for 5 minutes with 1 µg/mL of 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) in phosphate-buffered saline, and mounted in Clarion Mounting medium (Sigma-Aldrich). Images were acquired with LSM510 software (version 3.2) on an LSM 510 Meta Laser Confocal Microscope (Carl Zeiss Microscopy) using a Plan-Apochromat ×63/1.4 Oil DIC objective (Carl Zeiss Microscopy).
Flow cytometry
To measure trogocytosis, purified unlabeled PMNs were incubated at 37°C with 5% CO2 with PKH67-labeled CLL B cells at a 1:3 E:T ratio, in the presence or absence of 10 µg/mL of anti-CD20 or control antibodies. In some experiments, a dose-response curve was performed using 0.008 to 4 µg/mL of anti-CD20 antibodies. Cells were then analyzed at various times by flow cytometry with a FACSCanto II instrument (BD Biosciences, San Jose, CA). Gates to distinguish PMNs from CLL B cells were established using side scatter and PKH67 green fluorescence. The percentage of PMNs having acquired PKH67 from CLL B cells was then measured at different times by flow cytometry using a FACSCanto II instrument (BD Biosciences). These values were defined as percentage trogocytosis. Mean fluorescence intensity (MFI) of PKH67 on PMNs was also recorded. Negative controls included cocultures incubated in the absence of antibody (no monoclonal antibody [mAb]) or in the presence of the irrelevant CET antibody.
In experiments to measure CLL B-cell target cell death, purified PMNs were incubated at 37°C with 5% CO2 with unlabeled CLL B cells at a 1:3 E:T ratio, in the presence or absence of 10 µg/mL of anti-CD20 or the control antibody CET. At different times, anti-CD19 allophycocyanin, 7-aminoactinomycin D (7AAD) (BD Biosciences), and Cytocount calibration beads (Dako, Glostrup, Denmark) were added to the cocultures, and the percentages and absolute numbers of live or total CD19+ CLL B cells were measured using a FACSCanto II instrument (BD Biosciences).
To measure the loss of CD20 from CLL B-cell surfaces, purified PMNs were incubated with unlabeled CLL B cells at a 1:3 E:T ratio, in the presence or absence of 10 µg/mL of anti-CD20 or control antibody. After 3 hours of incubation at 37°C with 5% CO2, the amount of CD20 still detectable on the surface of CLL B cells was measured by adding an excess (10 µg/mL) of the same anti-CD20 antibody or CET control, followed by >20 µg/mL of monoclonal anti-human IgG fluorescein isothiocyanate antibody (clone HP-6017; Sigma-Aldrich) for detection. In some experiments, CLL B cells were incubated with anti-CD20 or control antibodies in the same conditions as described above, but without the addition of PMNs, to control for CD20 internalization. In all cases, samples were analyzed by flow cytometry using a FACSCanto II Instrument (BD Biosciences). The loss of CD20 expression was calculated as the percentage of MFI of samples incubated with the different anti-CD20 antibodies or CET, compared with the MFI of the control, which was incubated for the same length of time without antibody, but stained with the same antibodies.
In all experiments, PMNs from at least 3 different donors and B-cell targets from at least 3 different patients with CLL were used.
Statistical analysis
The data were analyzed using paired or unpaired Student t test, as appropriate; * represents P < .05; ** represents P < .01; and *** represents P < .001. The Pearson correlation coefficient was used to correlate different values with each other.
Results
PMNs mediate trogocytosis rather than phagocytosis of CLL B-cell targets
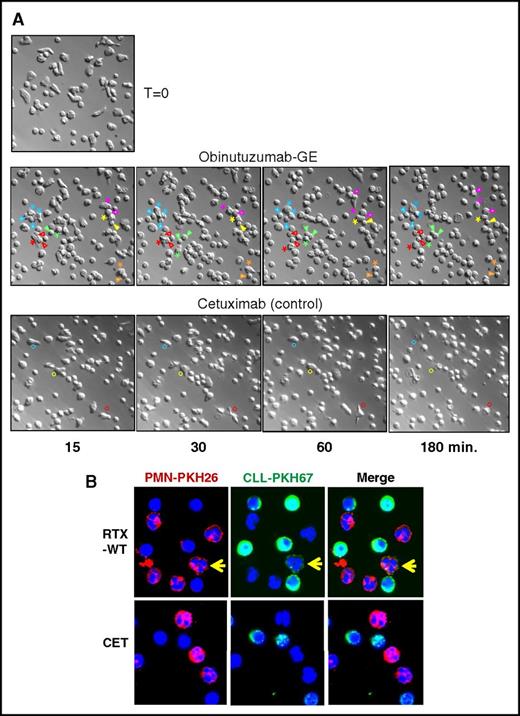
In order to clarify whether PMNs mediate trogocytosis or phagocytosis of opsonized primary CLL B cells, we performed live-cell time-lapse microscopy experiments. Purified PMNs from healthy donors were cocultured with CLL B-cell samples in the presence of the following anti-CD20 antibodies: wild-type rituximab (RTX-WT), glycoengineered rituximab (RTX-GE), or glycoengineered obinutuzumab (OBZ-GE). Cells were followed for up to 6 hours under the microscope. To our surprise, we could not detect any phagocytic event in up to 6 hours of time-lapse experiments, but only observed the repeated close contact between PMNs and anti-CD20–opsonized CLL B-cell targets, suggesting that trogocytosis rather than phagocytosis takes place. Figure 1A shows selected images of PMNs in contact with CLL B cells opsonized with OBZ-GE. The phase-contrast images obtained at the start of the experiment, before all CLL B cells had settled down to the bottom of the well, show the clear morphological differences between the larger, more irregular PMNs and the smaller, round CLL B-cell targets (Figure 1A; time [T] = 0). When OBZ-GE was added, PMNs (indicated by colored asterisks) began moving and repeatedly contacted the CLL B cells (indicated with colored arrows), but never phagocytosed them (Figure 1; supplemental Video 1, available on the Blood Web site). After 2 hours, the PMNs slowed down, and from 4 to 5 hours onward, they became granular and in most cases died (supplemental Video 1). In contrast, in the presence of the irrelevant anti-EGFR antibody CET as control, the PMNs made less frequent contact with CLL B cells and were less motile overall (Figure 1A; supplemental Video 2). Results obtained with RTX-WT and RTX-GE (supplemental Videos 3 and 4, respectively) were similar to those observed with OBZ-GE (supplemental Video 1). Ten independent time-lapse experiments were performed with the different anti-CD20 antibodies, following at least 200 individual PMNs, without detection of any phagocytosis. Finally, in order to verify that the PMNs were capable of phagocytosis under the conditions used, we performed identical time-lapse microscopy experiments using 4.5-µm antibody-coated beads as targets. We observed efficient phagocytosis of the 4.5-µm beads by about 50% of the PMNs within 2 hours (supplemental Video 5). This confirms that the PMNs used were competent for phagocytosis.
Microscopy images of PMNs cocultured with CLL B cells and anti-CD20 OBZ or CET control antibody. (A) Live-cell time-lapse microscopy experiments were performed with purified PMNs and CLL B cells at a 1:2 E:T ratio, cultured at 37°C in X-VIVO medium (Lonza) in the presence of 10 µg/mL of anti-CD20 OBZ-GE or CET control antibody. Time point 0 (T = 0): image taken at the start of the experiment before all CLL B cells had settled down to the bottom of the well. The other representative images were taken at ∼15, 30, 60, and 180 minutes after adding the indicated antibodies. Asterisks mark selected PMNs that repeatedly contacted the CLL B cells (indicated by arrowheads of the same color). Colored circles mark selected PMNs that made brief contact with CLL B cells in the control (CET) experiment. Original magnification ×400. The full videos of representative experiments in the presence of OBZ-GE (supplemental Video 1), CET (supplemental Video 2), RTX-WT (supplemental Video 3), RTX-GE (supplemental Video 4), and control beads (supplemental Video 5) are available on the Blood Web site. (B) Confocal microscopy images of PKH26-labeled PMNs (red) and PKH67-labeled CLL B cells (green) cocultured for 3 hours in the presence of 10 µg/mL of RTX-WT or CET control antibody. The yellow arrows indicate a representative red PMN that trogocytosed green PKH67 dye from CLL B cells. Original magnification ×630. Additional images are available (supplemental Figure 1).
Microscopy images of PMNs cocultured with CLL B cells and anti-CD20 OBZ or CET control antibody. (A) Live-cell time-lapse microscopy experiments were performed with purified PMNs and CLL B cells at a 1:2 E:T ratio, cultured at 37°C in X-VIVO medium (Lonza) in the presence of 10 µg/mL of anti-CD20 OBZ-GE or CET control antibody. Time point 0 (T = 0): image taken at the start of the experiment before all CLL B cells had settled down to the bottom of the well. The other representative images were taken at ∼15, 30, 60, and 180 minutes after adding the indicated antibodies. Asterisks mark selected PMNs that repeatedly contacted the CLL B cells (indicated by arrowheads of the same color). Colored circles mark selected PMNs that made brief contact with CLL B cells in the control (CET) experiment. Original magnification ×400. The full videos of representative experiments in the presence of OBZ-GE (supplemental Video 1), CET (supplemental Video 2), RTX-WT (supplemental Video 3), RTX-GE (supplemental Video 4), and control beads (supplemental Video 5) are available on the Blood Web site. (B) Confocal microscopy images of PKH26-labeled PMNs (red) and PKH67-labeled CLL B cells (green) cocultured for 3 hours in the presence of 10 µg/mL of RTX-WT or CET control antibody. The yellow arrows indicate a representative red PMN that trogocytosed green PKH67 dye from CLL B cells. Original magnification ×630. Additional images are available (supplemental Figure 1).
To confirm that membrane fragments, rather than whole cells, are transferred from CLL B cells to PMNs during trogocytosis, we labeled effector and target cells with 2 different dyes (red PKH26 for PMNs and green PKH67 for CLL B cells), incubated the cells with either RTX-WT, OBZ-GE, or the CET control antibody for 3 hours, and then analyzed the cells by confocal microscopy. As shown in Figure 1B and in supplemental Figure 1, dots of green fluorescence were detected on the membranes of red PMNs only in samples incubated with RTX-WT or OBZ-GE, but not with the CET control antibody. Trogocytosing PMNs are indicated by a yellow arrow. Nuclear staining (with 4′,6-diamidino-2-phenylindole) confirmed that the PMNs had not engulfed whole CLL B cells (Figure 1B; supplemental Figure 1).
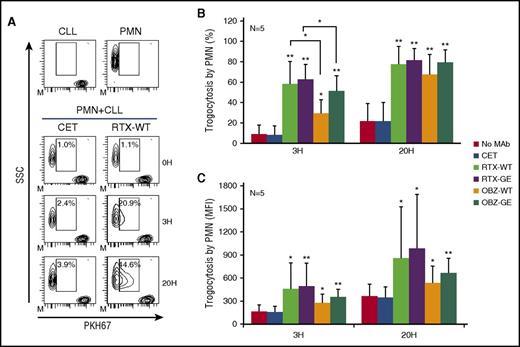
In order to better quantify trogocytosis on a larger number of cells, we then performed flow cytometry experiments using purified, unlabeled PMNs from healthy donors and CLL B cells stained with the membrane dye PKH67.12 A representative experiment performed with RTX-WT is shown in Figure 2A. The dot plots illustrate that CLL B cells and PMNs are easily distinguished by their respective side scatter properties. Furthermore, the intensity of the PKH67 fluorescence taken up by PMNs at 3 and 20 hours is considerably less than that observed on CLL B cells. This indicates a partial transfer of dye from CLL B cells to PMNs, confirming trogocytosis (passage of membrane fragments to the PMNs) rather than phagocytosis (uptake of whole CLL B cells by PMNs). Several conditions were analyzed, including different RTX-WT concentrations (0.001 to 100 µg/mL), E:T ratios (10:1; 3:1; 1:1, 1:3; 1:10), anti-CD20 antibodies or incubation times (2-6 hours), and the presence or absence of human serum. In no case could we detect any significant phagocytosis, but only trogocytosis. Trogocytosis was quantified as a percentage of PMNs becoming PKH67 positive (Figure 2B) or as an increase in the MFI of PKH67 on PMNs (Figure 2C). Indeed, in the presence of RTX-WT, a mean of 58% and 78% of PMNs had taken up membrane material from fluorochrome-labeled CLL B cells at 3 and 20 hours, respectively (Figure 2B), and MFI roughly doubled compared with controls (Figure 2C). All anti-CD20 antibodies mediated some trogocytosis. However, RTX was reproducibly more effective than OBZ, in the equivalent WT or GE versions, although this was not always statistically significant in this set of experiments, except in the case of percentage of trogocytosis at 3 hours (P < .5; Figure 2B). In contrast, the GE versions of both anti-CD20 antibodies were never statistically different from their respective WT versions (Figure 2B-C). In order to further analyze the efficiency of trogocytosis mediated by RTX compared with OBZ, we generated dose-response curves using 0.008 to 4 µg/mL of OBZ-GE and RTX-GE in parallel. We confirmed that trogocytosis was more effective with RTX-GE than OBZ-GE, whether trogocytosis was measured as MFI or as percentage of PKH67 on PMNs (P < .01) (Figure 3A, and data not shown).
PMNs mediate trogocytosis of CLL B cells opsonized with anti-CD20 antibodies. Purified PMNs were cocultured with PKH67-labeled CLL B cells at a 1:3 E:T ratio and 10 µg/mL of anti-CD20 antibodies RTX-WT, RTX-GE, OBZ-WT, OBZ-GE, or CET control antibody. At 3 (3H) or 20 hours (20H), partial transfer of fluorochrome (trogocytosis) from CLL B-cell membranes to PMNs was measured by flow cytometry, after gating PMNs according to side scatter (SSC). (A) Example of the gating approach showing CLL B cells and PMNs at time point 0 (0H) as well as in coculture experiments at various times in the presence of CET or RTX-WT. The gates show the percentages of PMNs engaged in trogocytosis. (B-C) Results of experiments performed with the indicated antibodies and in which either (B) percentage trogocytosis or (C) mean PKH67 fluorescence on gated PMNs were measured. Results are the means and standard deviations of 5 independent experiments. *P < .05; **P < .01.
PMNs mediate trogocytosis of CLL B cells opsonized with anti-CD20 antibodies. Purified PMNs were cocultured with PKH67-labeled CLL B cells at a 1:3 E:T ratio and 10 µg/mL of anti-CD20 antibodies RTX-WT, RTX-GE, OBZ-WT, OBZ-GE, or CET control antibody. At 3 (3H) or 20 hours (20H), partial transfer of fluorochrome (trogocytosis) from CLL B-cell membranes to PMNs was measured by flow cytometry, after gating PMNs according to side scatter (SSC). (A) Example of the gating approach showing CLL B cells and PMNs at time point 0 (0H) as well as in coculture experiments at various times in the presence of CET or RTX-WT. The gates show the percentages of PMNs engaged in trogocytosis. (B-C) Results of experiments performed with the indicated antibodies and in which either (B) percentage trogocytosis or (C) mean PKH67 fluorescence on gated PMNs were measured. Results are the means and standard deviations of 5 independent experiments. *P < .05; **P < .01.
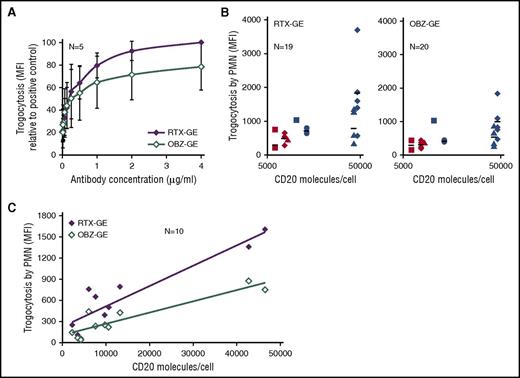
Trogocytosis efficiency correlates with CD20 occupancy and expression levels. Purified PMNs were cocultured with PKH67-labeled CLL B cells at a 1:3 E:T ratio for 3 hours. (A) CLL B cells were opsonized with 0.008 to 4 µg/mL of RTX-GE or OBZ-GE, and trogocytosis was measured at 3 hours by flow cytometry. The PKH67 MFI on PMNs, as a percentage of the positive control with 4 µg/mL RTX-GE, is shown. Data are the means and standard deviations of 5 experiments with 5 different CLL B-cell targets. (B) The PKH67 MFI values obtained in 20 experiments performed with PMNs from 9 healthy donors and 7 different B-cell targets (from 7 patients with CLL), opsonized with 10 µg/mL of RTX-GE (left panel) or OBZ-GE (right panel), were plotted against the number of CD20 molecules per CLL B cells (log scale). Each CLL B-cell target is represented by a different symbol or color. (C) The efficiency of trogocytosis by PMNs from a single healthy donor of 10 different CLL B-cell targets, all opsonized with 10 µg/mL of RTX-GE or OBZ-GE, was measured. The MFI of PKH67 on PMNs was plotted against the number of CD20 molecules per CLL B cell.
Trogocytosis efficiency correlates with CD20 occupancy and expression levels. Purified PMNs were cocultured with PKH67-labeled CLL B cells at a 1:3 E:T ratio for 3 hours. (A) CLL B cells were opsonized with 0.008 to 4 µg/mL of RTX-GE or OBZ-GE, and trogocytosis was measured at 3 hours by flow cytometry. The PKH67 MFI on PMNs, as a percentage of the positive control with 4 µg/mL RTX-GE, is shown. Data are the means and standard deviations of 5 experiments with 5 different CLL B-cell targets. (B) The PKH67 MFI values obtained in 20 experiments performed with PMNs from 9 healthy donors and 7 different B-cell targets (from 7 patients with CLL), opsonized with 10 µg/mL of RTX-GE (left panel) or OBZ-GE (right panel), were plotted against the number of CD20 molecules per CLL B cells (log scale). Each CLL B-cell target is represented by a different symbol or color. (C) The efficiency of trogocytosis by PMNs from a single healthy donor of 10 different CLL B-cell targets, all opsonized with 10 µg/mL of RTX-GE or OBZ-GE, was measured. The MFI of PKH67 on PMNs was plotted against the number of CD20 molecules per CLL B cell.
Because OBZ binds only half as many CD20 sites as does RTX, we wondered whether the more efficient trogocytosis mediated by RTX could be related to CD20 occupancy and to the CD20 expression levels on CLL B cells. We therefore analyzed the relationship between trogocytosis and CD20 expression levels in 20 experiments performed with 7 different CLL B cells expressing known levels of CD20 and PMN from 9 donors, in all cases using saturating concentrations of either RTX-GE or OBZ-GE (10 µg/mL). The results showed that there was much variability in the efficiency of trogocytosis of the same CLL B-cell targets by PMNs from different donors (Figure 3B, where each CLL B cell is represented by a different symbol). Also, the overall correlation between CD20 expression levels and trogocytosis in these experiments was not very significant (Pearson coefficients of 0.6 and 0.75 for RTX-GE and OBZ-GE, respectively [data not shown]). We therefore reasoned that, in addition to CD20 expression levels, other factors, such as Fc γ receptor polymorphisms on PMNs, may affect the efficiency of trogocytosis. Consequently, we collected the data obtained in different experiments using the same healthy PMN donor and CLL B cells from 10 different patients. The results show in this case a significant correlation between CD20 expression and the efficiency of trogocytosis by RTX-GE or OBZ-GE (Figure 3C) (Pearson correlation coefficients of 0.92 and 0.91, respectively).
We conclude that trogocytosis is mediated more efficiently by RTX-GE than OBZ-GE and correlates with CD20 expression levels, although additional factors are also likely to influence this mechanism.
Trogocytosis by PMN is accompanied by loss of CD20 from the CLL B-cell membrane
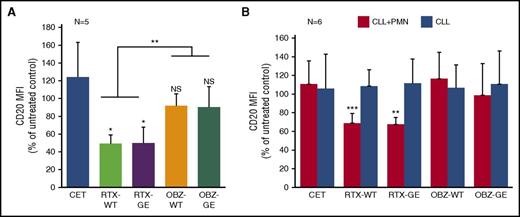
We then analyzed the possible loss of CD20 on the remaining CLL B cells after incubation with PMNs and different anti-CD20 or control antibodies, given that CD20 downmodulation is a hallmark of trogocytosis by monocytes/macrophages.8,13,14 By staining cells at 3 hours with excess corresponding anti-CD20 antibody and anti-human IgG fluorescein isothiocyanate, we could observe a 50% decrease in surface CD20 on CLL B cells after 3 hours of coculture with PMNs and RTX-WT or RTX-GE, compared with controls cocultured without antibody (or with the control antibody CET), confirming that trogocytosis of cell membrane proteins occurs (P < .05) (Figure 4A). In contrast, only 10% of CD20 downmodulation was observed in the presence of OBZ-WT or OBZ-GE, and this was not statistically significant (Figure 4A). Indeed, CD20 downmodulation was more marked with RTX than with OBZ in both the WT or GE versions (P < .01) (Figure 4A).
Trogocytosis is accompanied by CD20 downmodulation on CLL B-cell targets. Purified PMNs were cocultured with CLL B cells at a 1:3 E:T ratio in the presence of 10 µg/mL of anti-CD20 antibodies RTX-WT, RTX-GE, OBZ-WT, OBZ-GE, the CET control antibody, or no mAb. At 3 hours, surface CD20 still available was detected by adding an excess of the same anti-CD20 antibody followed by anti-human IgG fluorescein isothiocyanate. The results are the MFI (of fluorescein isothiocyanate) for CD20 on CLL B-cell targets, observed in wells treated with the indicated antibodies for 3 hours, as a percentage of that in equivalent wells without any antibody added. The results are the means and standard deviations of 5 independent coculture experiments with (A) PMNs and CLL B cells or (B) 6 separate experiments performed in the presence (black bars) or absence (gray bars) of PMNs. *P < .05; **P < .01; ***P < .001.
Trogocytosis is accompanied by CD20 downmodulation on CLL B-cell targets. Purified PMNs were cocultured with CLL B cells at a 1:3 E:T ratio in the presence of 10 µg/mL of anti-CD20 antibodies RTX-WT, RTX-GE, OBZ-WT, OBZ-GE, the CET control antibody, or no mAb. At 3 hours, surface CD20 still available was detected by adding an excess of the same anti-CD20 antibody followed by anti-human IgG fluorescein isothiocyanate. The results are the MFI (of fluorescein isothiocyanate) for CD20 on CLL B-cell targets, observed in wells treated with the indicated antibodies for 3 hours, as a percentage of that in equivalent wells without any antibody added. The results are the means and standard deviations of 5 independent coculture experiments with (A) PMNs and CLL B cells or (B) 6 separate experiments performed in the presence (black bars) or absence (gray bars) of PMNs. *P < .05; **P < .01; ***P < .001.
Some authors have previously shown that anti-CD20 antibodies can induce internalization of CD20.15,16 We therefore performed additional experiments under the same conditions as described above, but in the presence or absence of PMNs, to investigate whether the CD20 downmodulation was dependent on PMNs. In these experiments (n = 6), we observed that CD20 downmodulation indeed required the presence of PMNs (Figure 4B). Furthermore, we confirmed that RTX (WT or GE) induces significant CD20 downmodulation (P < .01), whereas OBZ (WT or GE) does not (Figure 4B), in agreement with the previous data (Figure 4A). The histograms of a representative CD20 downmodulation experiment, performed in the presence or absence of PMNs, are shown in supplemental Figure 2 to better illustrate this point.
Trogocytosis is not accompanied by CLL B-cell death
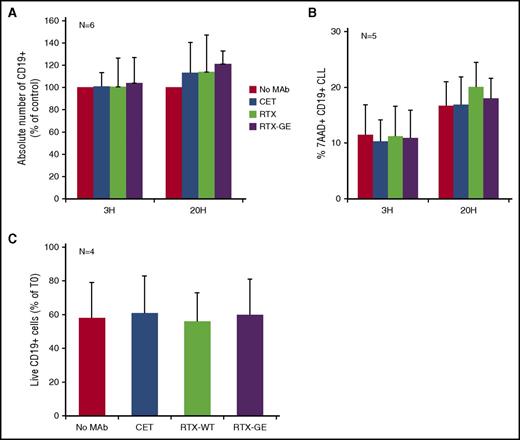
We finally measured the absolute number of CLL B cells at various times in the PMN coculture experiments. For these investigations, we used RTX-WT and RTX-GE because they were the strongest antibodies in the trogocytosis experiments and because OBZ induces homotypic adhesion of CLL B cells, potentially causing artifacts in these flow cytometry assays.17 We could not detect any significant decrease in the absolute number of CD19+ CLL B cells, after 3 or 20 hours of incubation in the presence of RTX-WT or RTX-GE, as compared with samples incubated with CET or no mAb (Figure 5A), confirming that no significant phagocytosis had taken place. In addition, we could not observe any significant induction of CLL B-cell death by staining with 7AAD (Figure 5B). Given that the removal of membrane fragments may induce cell death at relatively late time points,18 we also analyzed the absolute number and percentages of total and dead 7AAD+ CLL B cells after 3, 5, and 7 days of coculture with PMNs, in the presence or absence of RTX-WT or RTX-GE. No significant increase in target cell death was observed at any time point in the presence of any of the anti-CD20 antibodies tested (Figure 5C, and data not shown).
Trogocytosis by PMN is not accompanied by CLL B-cell death. Purified PMNs were cocultured with CLL B cells at a 1:3 E:T ratio and 10 µg/mL of anti-CD20 antibodies RTX-WT, RTX-GE, CET control antibody, or no mAb. At 3 (3H) and 20 hours (20H) (A-B) or at day 7 (C), anti-CD19 allophycocyanin, 7AAD, and calibration beads were added to measure the absolute number of total CLL B cells at different times (in panels A and C) or the percentage of dead 7AAD+ CD19+ CLL B cells (in panel B), respectively. The results are the means and standard deviations of 4 to 6 independent experiments.
Trogocytosis by PMN is not accompanied by CLL B-cell death. Purified PMNs were cocultured with CLL B cells at a 1:3 E:T ratio and 10 µg/mL of anti-CD20 antibodies RTX-WT, RTX-GE, CET control antibody, or no mAb. At 3 (3H) and 20 hours (20H) (A-B) or at day 7 (C), anti-CD19 allophycocyanin, 7AAD, and calibration beads were added to measure the absolute number of total CLL B cells at different times (in panels A and C) or the percentage of dead 7AAD+ CD19+ CLL B cells (in panel B), respectively. The results are the means and standard deviations of 4 to 6 independent experiments.
Discussion
In this report we demonstrate, using a variety of techniques, including live-cell time-lapse microscopy, confocal microscopy, and flow cytometry, that PMNs do not phagocytose CLL B-cell targets opsonized with different anti-CD20 antibodies, but rather mediate trogocytosis, ie, the transfer of membrane fragments from the target to the PMN effector. We and others had previously suggested that PMNs mediate phagocytosis of their CLL B-cell targets.4,6 However, these data were based on flow cytometry experiments, which could not easily distinguish between trogocytosis and phagocytosis. Here, the direct visualization in live-cell time-lapse microscopy experiments, as well as by confocal microscopy, clearly showed that PMNs do not actually engulf CLL B-cell targets, but rather come in repeated close contact with them. Although we cannot exclude that rare phagocytic events take place, clearly the predominant phenomenon observed here by different methods was trogocytosis.
Flow cytometry using membrane dyes allowed us to confirm the live-cell time-lapse microscopy data and more accurately quantify the transfer of membrane from CLL B cells to PMNs at different times. We observed that trogocytosis was not significantly different with glycoengineered antibodies compared with their equivalent wild-type, fully fucosylated antibodies, although some trend in favor of glycoengineered antibodies could be observed. These data suggest that CD32A plays a more important role than CD16B in this phenomenon. Previously, we have shown that PMN activation is more efficient with glycoengineered than wild-type anti-CD20 antibodies,4 suggesting that PMN activation may rely more directly on CD16B than trogocytosis,19,20 although CD32A may also be involved.21,22
Interestingly, the comparison between the type I (RTX) and type II (OBZ) anti-CD20 antibodies showed that RTX was significantly more effective in mediating trogocytosis than was OBZ, in either its glycoengineered or wild-type format. This was confirmed when complete dose-response curves were performed in parallel with RTX-GE and OBZ-GE. OBZ binds a slightly different CD20 epitope, and in a different conformation, compared with RTX. Furthermore, at saturation, CD20 occupancy by OBZ is half that of RTX.23 Thus, reduced trogocytosis by OBZ may be due to reduced CD20 occupancy by this antibody compared with RTX. In support of this hypothesis, our data show a strong correlation between CD20 expression levels on 10 different CLL B cells (measured as the number of CD20 molecules per cell) and trogocytosis efficiency, when PMNs from a single healthy donor were used as effector cells. This was true for both RTX-GE and OBZ-GE. In contrast, the efficiency of trogocytosis of the same CLL B-cell targets but by PMNs from different donors was relatively variable, suggesting that factors other than CD20 expression levels also influence trogocytosis. This is not surprising, given that the cell-cell interactions involved in trogocytosis are likely to involve the different Fc γ receptors expressed by PMNs (CD16B, CD32A), which are polymorphic, as well as other regulatory or inhibitory molecules. We conclude that CD20 expression and CD20 occupancy correlate with trogocytosis efficiency, but that additional factors likely also play a role. Further studies will be required to fully dissect this point.
Interestingly, the different binding modes of RTX and OBZ to CD20 have been shown previously to have several other important functional consequences: RTX induces translocation of CD20 into lipid rafts, complement activation, and CD20 internalization more efficiently than does OBZ.16,24,25 In contrast, RTX induces lower homotypic adhesion and less direct cell death than OBZ.15,19,26 Thus, we show for the first time, to our knowledge, that the mode of CD20 binding also modifies the efficiency of trogocytosis by PMNs.
Trogocytosis was accompanied by partial loss of CD20 from the CLL B-cell surface, as previously observed for macrophage-mediated trogocytosis.7,14 CD20 downmodulation under our study conditions was dependent on the presence of PMNs and therefore was not due to the previously described CD20 internalization.15 Our results are in agreement with previous data that showed faster and more efficient loss of CD20 by macrophage-mediated trogocytosis rather than by CD20 internalization.14 Loss of CD20 paralleled the transfer of membrane dye from CLL B cells to PMNs, because RTX was more efficient than OBZ in this case as well. Indeed RTX (WT or GE) resulted in 40% to 50% CD20 downmodulation at 3 hours, compared with 0% to 10% for OBZ-WT or OBZ-GE in different experiments.
We also observed here that PMNs did not induce significant cell death of CLL B-cell targets opsonized with RTX-WT or RTX-GE, either in short-term (20 hours) or long-term assays (up to 7 days). We did not specifically test OBZ antibodies in these assays, given that these are less efficient than the RTX versions in mediating trogocytosis, and they induce homotypic adhesion of B cells, which could lead to possible artifacts in the evaluation of CLL B-cell death by flow cytometry.17 Furthermore, OBZ may induce direct cell death in the absence of effector cells,19 and this would have complicated the interpretation of the results. The observed lack of cell death induction by RTX-WT and RTX-GE further confirms that no significant phagocytosis takes place, given that phagocytosis should have led to a decrease in the absolute number of CLL B-cell targets. Furthermore, it suggests that neither PMN activation,4 possibly leading to ROS production,5 nor the close contact between PMNs and their B-cell targets shown here, accompanied by removal of membrane fragments through trogocytosis, are sufficient to induce significant CLL B-cell death, at least under the conditions studied here. Thus, our data are different from those recently published that showed induction of breast cancer cell death by macrophages following trastuzumab-mediated trogocytosis.18 The reason for the difference is unclear, but it may be due to the different effector, target, or antibody used in these different studies.
Another important conclusion from our data is that a major mechanism of surface CD20 loss observed in vivo in patients with CLL treated with anti-CD20 antibodies27 may be due to trogocytosis by PMNs, in addition to that by monocytes and macrophages, which has already been well described.7,8,13 Further studies will be required to clarify the respective role of different cell types in CD20 loss in vivo. Similarly, the little or no CD20 downmodulation observed here in vitro in the presence of OBZ compared with RTX will need to be confirmed in vivo in patients, especially in view of the suggestion that CD20 downmodulation leads to resistance to anti-CD20 treatment in vivo.7,28
Finally, the reason for and/or function of trogocytosis in vivo, if any, is still unclear. We would like to put forward the hypothesis that trogocytosis represents an aborted phagocytosis. The live-cell time-lapse microscopy experiments suggested that PMNs repeatedly attempt to engulf their targets but fail to do so. This is in complete contrast with their capacity to rapidly and efficiently engulf artificial 4.5-µm beads opsonized with antibodies, as shown here. CLL B cells are approximately 10 µm in diameter and thus larger than the control beads used. Data from the literature,29 as well as our own preliminary experiments (R.V., unpublished observations), show that the capacity of PMNs to phagocytose opsonized beads depends largely upon bead diameter (optimal bead diameter appears to be 3-5 µm), and is much less efficient with larger 7-µm or 10- to 11-µm beads.29 Furthermore, CLL B cells are known to express inhibitors of macrophage-mediated phagocytosis, such as CD47,30 which may also inhibit phagocytosis by PMNs.31 Thus, relatively large objects like CLL B cells that also express inhibitors of phagocytosis, once opsonized with anti-CD20 antibodies, are clearly able to activate PMNs and induce attempts of phagocytosis, ie, close effector-target contact, but not complete phagocytosis. The repeated close contacts during failed phagocytosis may instead result in trogocytosis. Confirmation of this hypothesis will require more accurate studies using beads of different sizes and loaded with different amounts of antibody. Future studies will also need to determine whether PMN-mediated CD20 downmodulation through trogocytosis has functional consequences, especially on specific mechanisms of cytotoxicity that are induced by anti-CD20 antibodies, as suggested in some studies.7,32
Intriguingly, PMNs have been shown to play a role in the therapeutic activity of anti-CD20, as well as anti-HER2 and antimelanoma antibodies, in several in vivo mouse models.1-3 Thus, the mechanism of action of PMNs in vivo in the presence of anti-CD20 antibodies in these models is unclear at present but may be indirect, for example, through cytokine production.4,33 Alternatively, PMN activated in vivo in tissues may be able to mediate different functions compared with circulating PMNs, as studied here. Anti-CD20 antibodies also mediate other cell-mediated mechanisms, in particular, antibody-dependent cell-mediated cytotoxicity by natural killer cells, which is particularly active with glycoengineered mAbs.19,26,34 Also, phagocytosis of anti-CD20–opsonized CLL B cells by macrophages has been demonstrated by both flow cytometry and direct visualization.34-36 These latter mechanisms are therefore likely to be important for the therapeutic activity of anti-CD20 antibodies in vivo in patients with CLL and B-cell non-Hodgkin lymphoma.37
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the numerous patients and healthy volunteers who kindly donated peripheral blood for these experiments.
This work was supported in part by the Associazione Italiana Ricerca contro il Cancro (AIRC Investigator grant) (J.G.), Roche Innovation Center Zurich, Roche Pharma Research and Early Development, Schlieren, Switzerland, and Associazione Italiana contro le Leucemie-Linfomi e Myeloma–Sezione Paolo Belli, Bergamo, Italy.
Authorship
Contribution: R.V. and I.C. performed the experiments and analyzed the data; J.G. and M.F. supervised the experiments; C.K. provided reagents and funding and critically revised the data and manuscript; and J.G. and M.I. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: C.K. is an employee of Roche, the company that developed the RTX and OBZ anti-CD20 antibodies. J.G. has received research funding and a consultancy fee from Roche. The remaining authors declare no competing financial interests.
Correspondence: Josée Golay, Center of Cellular Therapy G. Lanzani, via Garibaldi 11-13, 24128 Bergamo, Italy; e-mail: jgolay@asst-pg23.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal