Key Points
Higher ibrutinib DI is associated with improved PFS, independent of del17p or TP53 mutation.
Ibrutinib hold for >1 week, often needed to manage adverse events, is associated with increased PFS events.
Abstract
Ibrutinib, an oral inhibitor of Bruton’s tyrosine kinase (BTK), at a once-daily dose of 420 mg achieved BTK active-site occupancy in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) that was maintained at 24 hours. It is unknown if intermittent interruption of ibrutinib therapy contributes to altered clinical outcomes. We therefore evaluated the effect of ibrutinib dose adherence on patient outcomes in the phase 3 RESONATE trial. The overall mean dose intensity (DI) was 95% with median treatment duration of ∼9 months. Pharmacokinetic assessment of ibrutinib exposure at 420-mg dose suggested similar exposure regardless of patient weight or age. As assessed by independent review committee, patients with higher DI experienced longer median progression-free survival (PFS) compared with those with lower DI regardless of del17p and/or TP53 status. Of 79 patients requiring a drug hold, treatment was restarted at the original dose in 73 (92%) patients. Mean duration of a missed-dose event was 18.7 days (range, 8-56). Patients missing ≥8 consecutive days of ibrutinib had a shorter median PFS vs those missing <8 days (10.9 months vs not reached). These results support sustained adherence to once-daily ibrutinib dosing at 420 mg as clinically feasible to achieve optimal outcomes in patients with previously treated CLL. The trial was registered at www.clinicaltrials.gov as #NCT01578707.
Introduction
Intermittent chemotherapy or chemoimmunotherapy has been the standard therapeutic approach for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).1-4 Ibrutinib, an oral Bruton’s tyrosine kinase (BTK) inhibitor, demonstrated improved progression-free survival (PFS), overall survival (OS), and overall response rate (ORR) compared with ofatumumab in a randomized phase 3 trial (RESONATE) of relapsed CLL/SLL.5
Using once-daily ibrutinib 420 mg in patients with CLL/SLL, BTK active-site occupancy was achieved at 4 hours and maintained at 24 hours.6 Transient reversal of treatment-related lymphocytosis and regrowth of lymph nodes occurred with intermittent dosing,6 suggesting rapid reversal of the biological effect. Additionally, fewer patients (26% to 51%) maintained optimal BTK occupancy (>95%) at lower daily ibrutinib doses (140-280 mg).7 The implications of interrupted therapy with ibrutinib are not known.
Sustained adherence to oral tyrosine kinase inhibitors is indicated as an important factor in achieving efficacy.8,9 Although once-daily dosing at 420 mg and lack of treatment interruptions could be needed for maximal benefit of ibrutinib, this has not been adequately addressed. Given that dose interruption/modification is warranted for treatment-related toxicity and invasive procedures, as recommended with ibrutinib use,10,11 we examined clinical impact of prolonged dose delays and reductions of ibrutinib in the RESONATE study.
Patients and methods
Design and interim analysis of RESONATE have been previously published.5
Treatment adherence to ibrutinib was measured by overall dose intensity (DIoverall) and DI in the first 8 weeks (DI8-week). DI was defined as the proportion of administered vs planned doses (for details on choice of 8-week interval and dosing, see supplemental Methods, available on the Blood Web site). The independent review committee (IRC)–assessed PFS and ORR in this retrospective analysis provide independent assessment of the impact of DI on outcomes.
The study was approved by the institutional review board or independent ethics committee at each institution.
Results
DI
All patients (N = 195) started on oral ibrutinib 420 mg once-daily, regardless of age, weight, or baseline comorbidities, and experienced similar exposure regardless of weight or age (supplemental Figure 1). The mean DIoverall was 95%, and the mean DI8-week was 96%, with a median treatment duration of ∼9 months. Although not statistically significant, patients with DI8-week below the mean (low DI) were older, had more advanced disease, and had more prior therapies compared with patients with DI8-week above the mean (high DI). Other baseline characteristics were similar between the 2 groups (supplemental Table 1).
Seventy-nine patients had dose holds for adverse events (AEs), 73 (92%) of whom restarted therapy at 420 mg consistent with United States Prescribing Information and European Union labels10,11 ; 5 patients restarted at a lower dose, and 1 did not restart therapy prior to data cutoff.
Eight patients had dose reductions because of AEs; 7 (3.6%) required a dose reduction to 280 mg, and 1 (0.5%) required a dose reduction to 140 mg. Four of 8 dose-reduced patients restarted or reattained the 420-mg dose. Diarrhea was the only AE resulting in dose reduction in >1 patient.
DI and survival outcomes
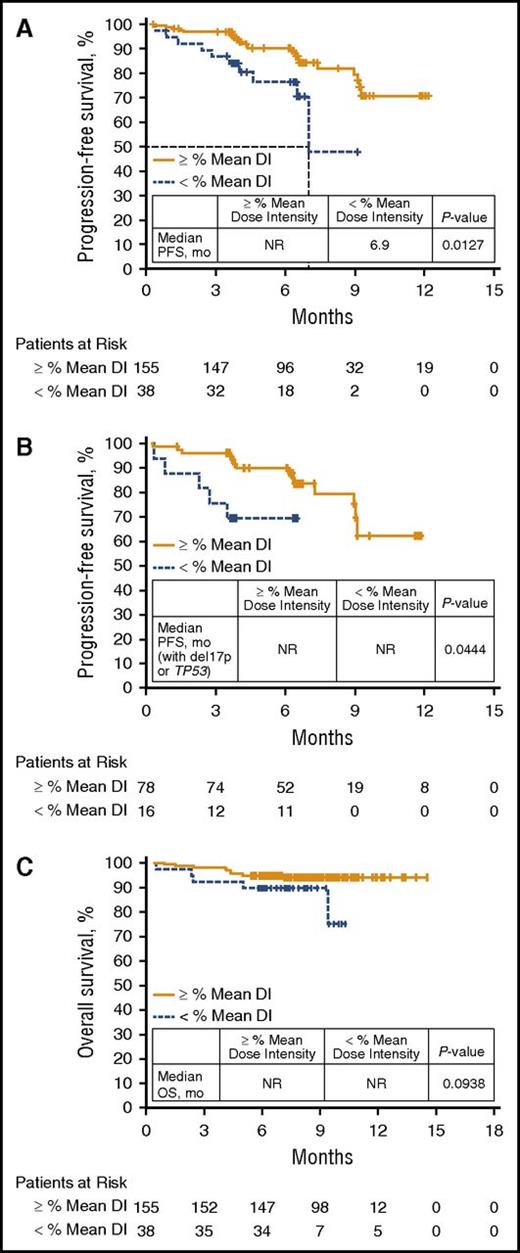
Fewer PFS events occurred in patients with high DI8-week vs those with low DI8-week (15% vs 26%), and similar results were observed for DIoverall (12% vs 33%, respectively). PFS was significantly longer in patients with high DI8-week vs low DI8-week (median PFS, NR [not reached] vs 6.9 months, P = .0127) (Figure 1A). Patients with del17p or TP53 mutation with high DI8-week also experienced fewer PFS events vs those with low DI8-week, with a significant difference in PFS (Figure 1B; P = .0444). Given the higher number of patients with low creatinine clearance and advanced-stage disease in the low DI8-week group (supplemental Table 1), a subgroup analysis was performed that showed no impact of these factors on PFS (supplemental Figure 2). An exploratory PFS analysis of DIoverall using a clinically relevant 80% cutoff revealed a marked difference in PFS for patients with DIoverall ≥80% vs those with DIoverall <80% (median NR vs 6.3 months, P < .0001; supplemental Figure 3).
Survival outcomes by mean DI. (A) PFS in all patients by 8-week mean DI. (B) PFS in patients with del17p or TP53 mutation by 8-week mean DI. (C) OS in all patients by 8-week mean DI.
Survival outcomes by mean DI. (A) PFS in all patients by 8-week mean DI. (B) PFS in patients with del17p or TP53 mutation by 8-week mean DI. (C) OS in all patients by 8-week mean DI.
Further, fewer deaths occurred in the high DI8-week vs low DI8-week group (5.8% vs 13.2%), with a trend toward improved OS (Figure 1C; P = .0938). The 6- and 12-month OS rates were 95% and 94% with high DI8-week vs 90% and 75% with low DI8-week.
Consecutive missed doses during entire treatment duration and survival outcomes per IRC
Fifty-eight patients who missed ≥8 consecutive days of ibrutinib 420 mg (supplemental Figure 4) had 78 missed-dose events, of which 54 events (69%) occurred primarily because of AEs, 10 (13%) because of surgeries, and 3 (0.4%) because of biopsies (supplemental Table 2). Mean duration of a missed-dose event in all treated patients was 18.7 days (range, 8-56). Patients with missed doses for ≥8 days experienced more IRC-assessed PFS events (17/57 [30%]) compared with patients missing <8 days (17/137 [12%]) with a significant difference in median PFS (10.9 months vs NR; P = .0151) (supplemental Figure 5).
IRC-assessed ORR
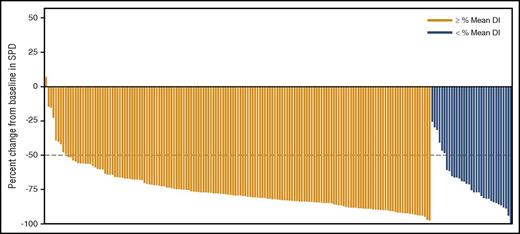
The ORR was 67% in patients with high DI vs 43% in those with low DI (P = .0080). Approximately 95% of patients with high DI experienced >50% reduction in the sum of the product of greatest diameters vs 82% of patients with low DI (Figure 2).
Maximum percent improvement from baseline in sum of the product of greatest diameters (SPD) based on mean DI between first dose and first disease assessment. Ninety-five percent of patients with high DI (above the mean) experienced a >50% reduction in SPD vs 82% of patients with low DI (below the mean).
Maximum percent improvement from baseline in sum of the product of greatest diameters (SPD) based on mean DI between first dose and first disease assessment. Ninety-five percent of patients with high DI (above the mean) experienced a >50% reduction in SPD vs 82% of patients with low DI (below the mean).
Post-IRC progression outcomes
With an additional 16-month follow-up (total median follow-up, 30.4 months; range, 0.33-36.5), 22/26 (84%) patients with IRC-confirmed progressive disease (PD) discontinued therapy. Fifteen of 26 (58%) patients who progressed by IRC also progressed clinically by investigator assessment prior to discontinuing ibrutinib. Ten of 26 patients who progressed by investigator assessment discontinued within 2 months of the independent PD event. Four of 26 patients with IRC-confirmed PD continue on ibrutinib at the time of analysis (supplemental Table 3).
Discussion
These retrospective analyses suggest that optimal adherence to the recommended ibrutinib dosage is associated with superior patient outcomes. Patients with higher mean DI demonstrated improved independently assessed PFS, higher ORR, and a trend toward improved OS. Ibrutinib hold for >1 week (∼3% reduction in DI) during the entire treatment duration was associated with increased PFS events. Because of the small number of dose-reduced patients (n = 8/195), it is difficult to draw an inference on the outcomes based on dose reduction.
Although IRC-confirmed PD represents independently confirmed disease, some of these events appear to be transient in nature following dose holds, with 11/26 (42%) patients being able to continue therapy without clinical progression for a period of time (>6.5 months). As such, following a dose hold, even with some increase in disease burden (lymph nodes or spleen), ibrutinib can provide continued disease control. It should be noted that dose interruptions/holds are clinically appropriate and inevitable in certain cases (eg, AE management, perioperative holds); however, our findings emphasize the importance of restarting and continuing the 420-mg dose, as soon as clinically appropriate, for optimal benefit. Additionally, therapy should not be discontinued without good cause, because the model for ibrutinib therapy is continuous treatment. Our data are supported by a recent report demonstrating that patients on ibrutinib who had treatment breaks >14 days during the first year experienced poor survival outcomes at ≥1 year.12
Further, ibrutinib should be initiated at 420 mg daily in all patients with CLL/SLL irrespective of weight or age, with similar exposure levels in these patients supporting this recommendation. Moreover, it may be important to avoid subtherapeutic drug levels, as this may promote resistance as observed in other tyrosine kinase inhibitor settings.13-15 Altogether, these data support the 420-mg dose and continuous administration schedule of ibrutinib in CLL/SLL that formed the basis of the superior efficacy observed in randomized studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was sponsored by Pharmacyclics LLC, an AbbVie Company. This manuscript was developed with editorial support from Swati Ghatpande and funded by Pharmacyclics LLC, an AbbVie Company.
Authorship
Contribution: P.M.B., J. C. Byrd, D.F.J., and S.S. contributed to the conception and design of the manuscript; all authors provided study materials or patients, contributed to collection and assembly of data, data analysis and interpretation, and contributed to review and revisions to manuscript content and final approval of manuscript.
Conflict-of-interest disclosure: P.M.B. has served as a consultant for Pharmacyclics and AbbVie and has received research funding from Pharmacyclics. J.R.B. has received honoraria from and has served as a consultant for Pharmacyclics, Janssen, Celgene, Gilead, Infinity, Genentech, and Pfizer; has received honoraria from Roche and Sun BioPharma; and has received travel reimbursement from Janssen, Gilead, Sun BioPharma, and Pfizer. P.H. has received honoraria from Pharmacyclics, Roche, Novartis, GlaxoSmithKline, Janssen, Gilead, AbbVie, and Pharmacyclics; has served as a consultant for Roche, GlaxoSmithKline, Janssen, Gilead, and AbbVie; and has received research funding from Roche, Novartis, GlaxoSmithKline, Janssen, Gilead, AbbVie, Celgene, and Pharmacyclics. S.O. has received honoraria and research funding and served as a consultant for Pharmacyclics and has received honoraria and served as a consultant for Janssen. J. C. Barrientos has served as a consultant for Gilead, AbbVie, and Janssen and has received research funding from AbbVie and Gilead. N.M.R. has served as a consultant for AbbVie, Gilead, Infinity, and Celgene. S.C. has served as a consultant for Janssen and Pharmacyclics and has received research funding from AbbVie and Pharmacyclics. S.P.M. has received honoraria from and has served as a consultant for Roche, AbbVie, Janssen, Gilead, and GlaxoSmithKline; has received research funding from Roche, AbbVie, and Janssen; and has participated in the speaker’s bureau for Roche, AbbVie, Janssen, and Gilead. U.J. has received honoraria from, served as a consultant for, and received travel reimbursement from Janssen. R.R.F. has received honoraria from, has served as a consultant for, and has participated in the speaker’s bureau for Pharmacyclics. F.C. has received honoraria from Janssen, Mundipharma, Gilead, and Karyopharm; has served as a consultant for Janssen and Gilead; has received research funding from Janssen and Celgene; and has received travel reimbursement from Janssen, Mundipharma, and Roche. M.M. has served as a consultant for and has received honoraria from Roche, Gilead, Janssen, and GlaxoSmithKline. C.D. has served as a consultant for and received honoraria from Roche, Gilead, Janssen, Sanofi, and AbbVie; has provided expert testimony for Gilead; and has received travel reimbursement from Roche and Gilead. T.R. has served as a consultant for and received honoraria and research funding from Pharmacyclics, Janssen, and AbbVie. J.M.P. has served as a consultant for Pharmacyclics and Gilead. J.A.B. has served as a consultant for Jansen and Portola, has received research funding from Pharmacyclics and Gilead, and has received travel reimbursement from Roche and Janssen. S.S., J.S., G.C., and D.F.J. are employees of Pharmacyclics LLC, an AbbVie Company, and have stock ownership with AbbVie. J.S. also has stock ownership with Global Blood Therapeutics and Portola Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Paul M. Barr, James P. Wilmot Cancer Center, University of Rochester Medical Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: paul_barr@urmc.rochester.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal