In this issue of Blood, Yang et al explore the effects of the uremic toxin indoxyl sulfate (IS) triggering platelet hyperactivity in chronic kidney disease (CKD). In their mouse model of disease, the aging suppressor protein Klotho acts as an antidote for the toxin effects.1
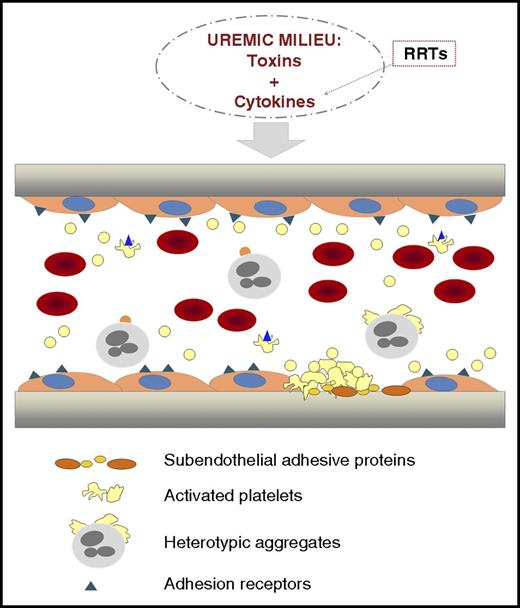
Illustration representing a longitudinal section of a vascular vessel. In CKD, toxins and cytokines are present in the circulation. RRTs, although necessary, contribute to activate circulating blood cells with the release of cytokines that enrich the toxic environment. Chronic inflammation and oxidative stress coexist with endothelial dysfunction in CKD. The hematocrit is decreased because of the anemia. Activated platelets circulate and form heterotypic aggregates with monocytes. Platelet hyperactivity contributes to thrombus formation. From Yang et al, the uremic toxin indoxyl sulfate seems to play a crucial role in the activation of these processes, and Klotho protein could be a promising strategy to prevent them.
Illustration representing a longitudinal section of a vascular vessel. In CKD, toxins and cytokines are present in the circulation. RRTs, although necessary, contribute to activate circulating blood cells with the release of cytokines that enrich the toxic environment. Chronic inflammation and oxidative stress coexist with endothelial dysfunction in CKD. The hematocrit is decreased because of the anemia. Activated platelets circulate and form heterotypic aggregates with monocytes. Platelet hyperactivity contributes to thrombus formation. From Yang et al, the uremic toxin indoxyl sulfate seems to play a crucial role in the activation of these processes, and Klotho protein could be a promising strategy to prevent them.
Hemostasis is in a delicate equilibrium in CKD patients. Deficient hemostasis coexists paradoxically with accelerated atherosclerosis and an enhanced thrombotic risk. Uremic bleeding is multifactorial and has been attributed to platelet dysfunction, impaired platelet–vessel wall interactions, and altered rheological properties of the blood flow. Treatment with erythropoiesis-stimulating agents was introduced in the mid-1980s with a very favorable impact, not only in reducing the frequency of bleeding, but also in improving patients’ overall quality of life.2 The prothrombotic state in CKD may be related to an imbalance between coagulation factors and coagulation inhibitors, decreased fibrinolytic activity, platelet hyperactivity, and endothelial dysfunction. At present, although the incidence of bleeding is apparently decreasing, the thrombotic complications have become the main causes of mortality in this population.
Accelerated atherothrombosis in CKD has a complex etiology (see figure). Endothelial dysfunction coexists with a chronic inflammatory state and oxidative stress in uremic patients.3,4 In the development of these pathological processes, there are at least 2 components: humoral and cellular. The humoral component consists of the presence of uremic toxins and those factors released by the activation of blood cells. The uremic toxins are present in patients with CKD, independent of treatment with renal replacement therapies (RRTs), and alter the function of the different cell populations involved in hemostasis (endothelial cells, platelets, and leukocytes). These activated cellular elements release cytokines that enrich the humoral component. In addition, RRTs themselves promote cell activation and further production and release of cytokines, further propelling the inflammatory reaction. Uremic toxins can be classified into 3 main groups: small water-soluble compounds, middle molecules, and protein-bound solutes, with the common characteristic of being difficult to eliminate by conventional RRTs.5 IS, a protein-bound uremic toxin derived from the amino acid tryptophan, is produced by the intestinal flora and is 1 of the clinical factors thought to contribute to CKD progression.
Yang et al conducted a series of experiments to examine IS triggering of platelet activation and thrombosis both in vitro and in vivo in a mouse model. The authors present convincing data of platelet hyperactivity caused by IS, as demonstrated by an enhanced response to the platelet agonists thrombin and collagen; increased P-selectin expression; release of platelet microparticles; formation of heterotypic platelet-monocyte aggregates; and higher platelet adhesion in a thrombosis model of carotid artery occlusion. The platelet effect of IS, which is an activator of oxidative stress, seems likely to be mediated through the production of reactive oxygen species (ROS) and the activation of the inflammation-related protein p38 MAPK.
Furthermore, the authors show that Klotho protein modulates the effect of IS on platelet hyperactivity and thrombus formation, protecting against IS-induced atherosclerosis in apoE null mice. The role of Klotho in this setting is intriguing. Klotho is known to be an aging suppressor protein that acts as a scavenger for ROS overproduction. It is highly expressed in the kidney, and CKD is a state of Klotho deficiency, with negative systemic effects on numerous organs, including the cardiovascular system. Results provided by Yang et al, together with recently generated evidence,6 indicate that this protein could be a future prophylactic and therapeutic target to modify or prevent the progression from acute to CKD with its associated cardiovascular risk.
There are a number of publications exploring the effect of IS on different cell types, such as endothelial cells, myoblast cells, and smooth muscle cells. IS is a protein-bound toxin, and high free concentrations of these compounds and/or excessively low albumin concentrations may distort the interpretation of the results obtained and therefore overestimate the toxic impact of IS.7
The findings by Yang et al give insight into the pathogenesis of CKD-associated thrombosis and accelerated atherosclerosis and suggest new strategies for their treatment. Correction of Klotho deficiency may be a promising strategy to prevent, retard, and decrease the burden of comorbidity in CKD. The results obtained through this study offer different scenarios for promising future research. Translational studies on humans are needed to confirm the results obtained in the mouse model. CKD is a very complex pathological situation, and alterations occurring in those cells constantly exposed to the humoral blood components are multifactorial. IS is just 1 of the multiple toxins present in the circulation of CKD patients. There is emerging evidence on the influence of gut bacteria on the production of uremic toxins and the development of CKD.8 The search for new strategies to diminish the concentrations of these uremic toxins will benefit the management of CKD and end-stage renal disease patients in which there is still residual renal function.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal