In this issue of Blood, Chen and colleagues demonstrate an unanticipated role for the contact system in a murine model of Alzheimer disease (AD). The study bolsters the key role of vascular dysfunction in AD by identifying factor XII (FXII) activation as a new molecular player in AD pathogenesis through mechanisms linked to cerebral fibrin deposition, neuroinflammation, and neuronal damage.1
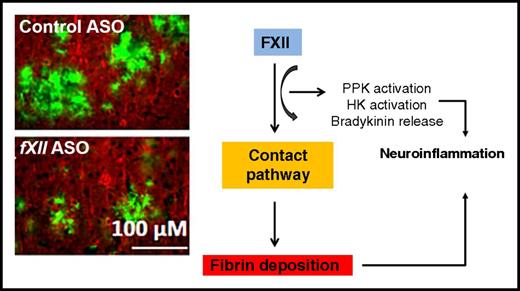
Coagulation FXII has an essential role in inflammation-mediated neuronal damage and cognitive impairment in an AD mouse model. Similar to depletion of fibrinogen, depletion of FXII decreases neuroinflammatory responses and subsequent brain pathology. Histological images have been adapted from Figure 6 in the article by Chen et al that begins on page 2547.
Coagulation FXII has an essential role in inflammation-mediated neuronal damage and cognitive impairment in an AD mouse model. Similar to depletion of fibrinogen, depletion of FXII decreases neuroinflammatory responses and subsequent brain pathology. Histological images have been adapted from Figure 6 in the article by Chen et al that begins on page 2547.
Cerebrovascular pathology and neuroinflammation are central to AD pathogenesis.2 Accumulating evidence overwhelmingly points to the coagulation cascade as a mechanism linking these two temporally closely related pathologic processes in AD. Activation of coagulation has been implicated in driving detrimental vessel wall pathology and neuroinflammatory cascades in AD and other neurodegenerative disorders. Fibrin derived from the thrombin-mediated conversion of fibrinogen is the end product of the coagulation cascade. Multiple studies in humans and experimental animal models have shown that fibrin is causally linked with neuroinflammation.3 Primarily because of its unique binding to specific integrin receptors on inflammatory cells, fibrin mediates, controls, and sometimes triggers neuroinflammation.4,5 Fibrin is, therefore, implicated in the pathogenesis of various neurological diseases with an inflammatory component, including vasculitis, stroke, brain trauma, and multiple sclerosis.3 Importantly, fibrin has a central role in AD pathogenesis by promoting neurodegeneration and neuroinflammation and impairing cognitive function.6-8 Indeed, inhibiting the interaction between amyloid beta (Aβ) and fibrinogen rescues cerebrovascular occlusion and cognitive impairment in vivo.6 It remains, however, unclear how the upstream components of the coagulation cascade affect neuroinflammatory disease pathogenesis.
In their study, Chen et al further elucidated the role of FXII in AD pathogenesis. FXII drives the contact system, which initiates procoagulant and proinflammatory pathways by activating FXI and plasma prekallikrein (PPK), respectively. Previous elegant studies by the authors showed that the FXII-mediated contact pathway is activated in AD patients by Aβ, thereby stimulating the conversion of fibrinogen to the inflammatory fibrin.7 Given the dual procoagulant and proinflammatory roles of FXII, the authors argued that this system could be activated at early AD stages. Using the TgCRND8 AD mouse model harboring three mutations in the amyloid precursor protein pertinent to Aβ pathology, the authors found a temporal correlation between activation of the inflammatory branch of the contact pathway and the onset of neuroinflammation, as indicated by increased cleavage of high-molecular-weight plasma kininogen (HK). Strikingly, by depleting FXII, the authors showed that plasma HK cleavage, neuroinflammation, cerebral fibrin deposition, and neurodegeneration were reduced and cognitive function was improved in the mice studied. These results provide further evidence for the role of the coagulation cascade in AD and suggest that individual members of the cascade, including FXII, may be potential therapeutic AD targets.
Given the synergy between neuroinflammation and vascular pathology in AD pathogenesis, this study raises the important question how they are linked mechanistically. The authors asked first whether a temporal correlation exists between HK cleavage/contact system activation and AD progression. By measuring plasma intact HK (HKi) levels in TgCRND8 mice at different disease stages, they found that these were already decreased in 3-month-old mice, indicating increased HK cleavage starting at early Aβ pathology. These actions were accompanied by increased astrocytosis and microgliosis, although the latter became significant only at 6 months of age. Whether this differential temporal regulation between the HK and microgliosis data indicates that the temporal correlation between changes in HK cleavage and onset of microglia activation is actually less pronounced than suggested by the authors or is due to the authors’ use of Iba-1 instead of the more sensitive CD11b as a marker of microglia activation in these specific experiments is unclear. Interestingly, by depleting plasma FXII with antisense oligonucleotide (ASO)–mediated messenger RNA knockdown, levels of both HKi and PPK in the treated transgenic mice were similar to those in the wild-type controls. These findings indicate that FXII activation indeed mediates the proinflammatory branch of the contact system in an AD-like mouse model, thus promoting neuroinflammatory responses.
In a subsequent set of experiments, the authors treated 4-month-old TgCRND8 mice with FXII ASO for 2 months. This treatment paradigm reduced cerebral inflammation and fibrin deposition but, importantly, also decreased neuronal damage and improved cognitive performance in the Barnes maze and contextual fear-conditioning paradigm. These results underline the strong links that exist among the coagulation cascade, neuroinflammation, and neuronal damage (see figure).3 Nevertheless, the nature of the fibrin deposits found in this study (ie, whether they are intravascular clots, perivascular deposits, and/or actual parenchymal deposits associated with Aβ) cannot be evaluated from the data. Future studies are also warranted to determine the exact temporal effects of FXII and the downstream members of the coagulation cascade on the specific components of the neuronal circuitry.
Given that FXII binds to endothelial cells via the gC1q receptor, could FXII extravasate into the brain and bind to central nervous system (CNS) proteins?9 Do the different Aβ species in the brain activate FXII to a different extent than those in the circulation? These are some pertinent questions raised by the exciting findings of this study. As the authors suggest, activation of FXII ultimately leads to release of bradykinin, which may increase blood–brain barrier permeability. Therefore, entry of FXII into the CNS, similar to that which is well established for fibrinogen, is likely. Determining the presence and fate of FXII in the CNS may thus reveal unexpected and highly interesting novel binding interactions between FXII and CNS proteins. As for the latter question, the group previously showed in vitro that oligomeric Aβ42 in plasma activates FXII more potently than that observed for its monomeric and fibrillar forms.7 However, it is not known how the aggregation state of Aβ42 and possibly of other Aβ species determines the activation of FXII in vivo, especially in the critical brain milieu.
This exciting study by Chen and colleagues positions FXII among the key players of the coagulation cascade that contribute to AD pathogenesis by instigating neuroinflammatory responses. It shows encouraging promise for FXII and other members of the coagulation cascade as potential, highly needed novel therapeutic targets in neurodegenerative diseases.
Conflict-of-interest disclosure: K.A. served as a consultant for Lundbeck and is a named inventor on patents and patent applications related to fibrin. M.M. declares no competing financial interests.