Key Points
Numerous empty HSC niches, located distantly from filled niches, are available for engraftment and proliferation in bone marrow.
Presumptive niches for granulocyte/macrophage progenitors appear to be filled in bone marrow.
Abstract
Hematopoietic stem cells (HSCs) reside in and are maintained by special microenvironments, termed niches. It is assumed that the HSC niche space remains occupied by endogenous cells and that myelosuppressive conditioning is required to achieve high levels of HSC engraftment. We herein demonstrate that upon the transplantation of very large numbers of purified HSCs into normal mice not exposed to myeloablation, donor HSCs engrafted in niches distant from filled HSC niches without replacing host HSCs and subsequently proliferated and generated hematopoietic progenitors, leading to marked increases in the overall HSC numbers in bone marrow. Additionally, stem cell factor that is produced by CXC chemokine ligand 12–abundant reticular cells is involved in HSC engraftment. In contrast, host granulocyte/macrophage progenitors (GMPs) were replaced by the progeny of transplanted donor HSCs, and overall GMP numbers remained unchanged. Thus, inconsistent with the classical concept, numerous empty HSC niches are available for engraftment and proliferation in bone marrow.
Introduction
All lineages of blood cells are generated from relatively rare hematopoietic stem cells (HSCs), which reside in and are maintained by special microenvironments, known as niches, in bone marrow throughout life.1-7 Recent studies confirmed that cells in the classically defined HSC population contribute to steady-state hematopoiesis.8,9 Transplantation of HSCs into a host can reconstitute and sustain healthy hematopoiesis10 and offers a curative approach for patients with hematopoietic malignancies. In addition, HSC transplantation has shown promising potential to cure a number of nonmalignant, life-threatening blood disorders, including hemoglobinopathies, congenital bone marrow failure syndromes and immunodeficiencies, autoimmune diseases, and AIDS. However, it has been assumed that an increase in the number of HSCs is limited by the occupancy of their niches by endogenous HSCs and that most transplanted HSCs fail to engraft in the bone marrow without myelosuppressive host conditioning, which causes severe toxicity, because niches for HSCs are filled.11-16
Previous studies reported that the transplantation of whole bone marrow cells led to greater levels of long-term HSC engraftment, even in the absence of myelosuppressive conditioning.17-20 However, upon the transplantation of purified primitive hematopoietic cells or HSCs into unconditioned animals, only a small fraction of HSC niches (∼0.1% to 10%), in which HSC replacement occurred, were available for engraftment.14-16,19 These findings suggest that HSC niches are filled and that presumptive non–stem cell facilitator cells, which exist within transplanted bone marrow cells, are required for higher levels of HSC replacement by donor HSCs in the absence of myelosuppressive conditioning.
Consistent with this hypothesis, various rare nonhematopoietic cell populations, including osteoblasts lining the bone surface21,22 and periarteriolar cells, including CD45−lineage marker (Lin)−platelet-derived growth factor receptor α+Sca-1+ cells,23 nestin+ neural/glial antigen 2+ mesenchymal stem cells,24-26 and nonmyelinating Schwann cells,27 have been reported to create unique niches for HSCs. However, recent studies have shown that ablation of osteoblasts in vivo did not reduce the numbers of HSCs28,29 and that most HSCs are not associated with the bone surface and/or arteries.30 Other studies have shown that macrophages expressing α-smooth muscle actin were located adjacent to HSCs31 and that ∼20% of HSCs were in contact with megakaryocytes, suggesting that these rare hematopoietic cells create HSC niches.32-34
On the other hand, more abundant populations of nonhematopoietic cells, including sinusoidal endothelial cells,35-39 and a population of adipo-osteogenic progenitors, called CXC chemokine ligand (CXCL)12–abundant reticular (CAR) cells, which strongly overlap with leptin receptor–positive (LepR+) cells,38-42 have been shown to create a niche for HSCs. Approximately 60% of HSCs are in contact with sinusoidal endothelial cells.35 When stem cell factor (SCF) was conditionally deleted from endothelial cells, the number of HSCs was reduced.35,38 Additionally, it has been shown that more than 94% of HSCs are in contact with CAR (LepR+) cells30,40 and that all CAR (LepR+) cells homogeneously express substantially higher levels of CXCL12, SCF, and the transcription factor Foxc1, which are essential for maintaining HSCs, than other bone marrow cell populations.38,40,42 Short-term ablation of CAR cells in vivo using a diphtheria toxin receptor–mediated cell knockout technique led to a severe reduction in the numbers of hematopoietic stem and progenitor cells.41 When SCF was conditionally deleted from CAR (LepR+) cells, the number of HSCs was severely reduced in bone marrow.38 These results suggest that CAR (LepR+) cells as well as endothelial cells are a major cellular component of HSC niches. Of note, the numbers of CAR (LepR+) cells and sinusoidal endothelial cells were markedly larger than HSC numbers.30,38,40 These findings do not rule out the possibility that a small population of CAR (LepR+) cells and/or sinusoidal endothelial cells create unique saturable niches for HSCs but rather suggest the existence of empty HSC niches and prompted us to address this issue. It is important to know if the availability of niches is a limitation of HSC expansion and engraftment in order to understand HSC behavior and develop therapeutic strategies using HSCs for a number of diseases.
We herein demonstrate that upon the transplantation of very large numbers of purified HSCs into normal mice that were not exposed to irradiation or other myeloablation, many donor HSCs engrafted into empty niches that were distant from niches in which endogenous host HSCs resided, and subsequently proliferated and generated hematopoietic progenitors. Our results prompt the reevaluation of a long debate on HSC niche saturation, indicate that the rarity of HSCs is not a consequence of space limitations, and suggest that we do not have to consider the availability of HSC niches to achieve efficient HSC engraftment in clinical bone marrow transplantation.
Methods
Mice
Col1A1–histone 2B (H2B)–green fluorescent protein (GFP) fusion protein, Rosa26-M2-rtTA (TetOP-H2B-GFP),43 SCFf/f,38 and Lepr-Cre44 mice (all from the Jackson Laboratory) were backcrossed onto a C57BL/6-CD45.2 or C57BL/6-CD45.1 background. Recipient mice used were 8- to 11-week-old male CD45.1 × CD45.2 C57BL/6 wild-type, TetOP-H2B-GFP, SCFf/f or Lepr-Cre;SCFf/f mice. Donor mice used were age- and sex-matched, congenically distinguishable, CD45.2 C57BL/6 wild-type or TetOP-H2B-GFP mice. For H2B-GFP expression, TetO-H2B-GFP mice received doxycycline (Sigma-Aldrich D9891, 2 mg/mL supplemented with 1% sucrose in drinking water) for 6 weeks as described previously.43 Mice were housed in a specific pathogen-free facility, and all animal experiments were performed in accordance with approved protocols of the Institutional Animal Care and Use Committees at Kyoto University and Osaka University.
Flow cytometry
Bone marrow cells were obtained by flushing femurs and tibias. All staining was performed in 2% fetal calf serum with commercially prepared antibodies. Dead cells were excluded by staining with propidium iodide. Flow cytometric experiments and cell sorting were performed using a FACSAria I or a FACSAria II-SORP (BD Biosciences). HSCs were defined as CD34−CD150+CD48−Lin−Sca-1+c-kit+, granulocyte/macrophage progenitors (GMPs) were defined as Lin−Sca-1−c-kit+CD34+FcγRII/IIIhi, and common lymphoid progenitors (CLPs) were defined as Lin−CD19−Flt3+IL-7Rα+. Lineage markers were composed of CD3, CD11b, B220, Gr-1, and Ter119. The antibodies used are listed in supplemental Table 1 (available on the Blood Web site).
Isolation of HSCs
Bone marrow cells obtained by flushing femurs and tibias of donor mice were first incubated with purified monoclonal antibodies to lineage markers (B220, CD3, CD4, CD8, CD11b, Gr-1, and Ter119). After washing, Lin− cells were obtained by removing labeled cells by 2 consecutive incubations with anti-rat immunoglobulin G–coated M450 Dynabeads (Thermo Fisher), and then stained with fluorochrome-conjugated antibodies against CD34, CD48, Sca-1, c-kit, and lineage markers. CD34−CD48−Lin−Sca-1+c-kit+ HSCs were sorted from Lin− cells on a FACSAria I and a FACSAria II-SORP at a rate of 2500 to 3500 cells per second. Typically, 2.5 × 105 purified CD34−CD48−Lin−Sca-1+c-kit+ HSCs were obtained from bone marrow of 210 mice, and 6.5 × 108 purified Lin− cells were obtained from bone marrow of 120 mice.
HSC transplantation
Unconditioned CD45.1 × CD45.2 C57BL/6 wild-type mice were intravenously transplanted with 2500, 1 × 104, 2 × 104, 3 × 104, 4 × 104, or 6 × 104 purified HSCs or 1.6 × 108 purified Lin− cells from CD45.2 C57BL/6 mice given as a single bolus, with a sum total of 2.5 × 105 purified HSCs from CD45.2 C57BL/6 mice given over the course of 6 injections at 3-day intervals, or with a sum total of 6.5 × 108 purified Lin− cells from CD45.2 C57BL/6 mice given over the course of 4 weekly injections. At 13 to 16 weeks after transplantation, bone marrow cells from femurs and tibias of wild-type recipients were harvested and analyzed. Unconditioned CD45.1 × CD45.2 control or Lepr-Cre;SCFf/f mice were intravenously transplanted with 4.0 × 104 purified HSCs stained with PKH26 (Sigma-Aldrich) or 1.6 × 108 purified Lin− cells from CD45.2 C57BL/6 mice given as a single bolus. Forty-eight hours or 16 weeks after transplantation, bone marrow cells from femurs and tibias of control or Lepr-Cre;SCFf/f recipients were harvested and analyzed.
Competitive repopulation assay, location of transplanted and endogenous HSCs, immunohistochemical analysis, and statistical analysis
Detailed procedures are described in the supplemental Methods.
Results
Transplanted HSCs engraft into nonmyeloablated bone marrow without the replacement of endogenous HSCs
In order to more clearly quantify the absolute numbers of transplanted HSCs in unconditioned recipient bone marrow, we transplanted larger numbers of purified HSCs into unconditioned mice not exposed to myeloablation compared with previous studies.14-16 Varying numbers of cells in the purified population of HSCs, defined as the CD34−CD48− subset of lineage marker (Lin) −Sca-1+c-kit+ (LSK) cells (supplemental Figure 1),30,35 from the bone marrow of 8- to 11-week-old CD45.2 mice, were intravenously injected into 8- to 11-week-old unconditioned congenic CD45.1 × CD45.2 mice, and the donor chimerism and absolute numbers of hematopoietic cells, including HSCs, were measured 13 to 16 weeks after transplantation. Flow cytometric analyses revealed that the donor HSC chimerism in the bone marrow increased markedly at doses of 2500 to 2.5 × 105 transplanted donor HSCs (Figure 1A). The absolute number of donor phenotypic HSCs (CD34−CD150+CD48−LSK) increased after the transplantation of HSCs in a linear dose-dependent manner (Figure 1B), whereas the number of endogenous phenotypic HSCs remained largely unchanged (Figure 1C and data not shown). When 2.5 × 105 purified HSCs, representing ∼390% of the total number of HSCs in an adult mouse,45 were transplanted over the course of 6 injections at 3-day intervals, whereas the number of endogenous phenotypic HSCs was similar, the total number of phenotypic HSCs in the bone marrow was approximately twofold higher than that in untransplanted animals at 24 weeks of age (Figure 1C). Thus, many transplanted HSCs engrafted into host bone marrow without HSC replacement. We then estimated the engraftment of functional HSCs using repopulating units (RUs) based on a competitive long-term repopulation assay. After the transplantation of 2.5 × 105 purified HSCs from the bone marrow of 8- to 11-week-old CD45.2 mice into unconditioned congenic CD45.1 × CD45.2 mice, whereas the number of endogenous RUs was similar, the total number of RUs in the bone marrow was approximately twofold higher than that in untransplanted animals at 24 weeks of age (Figure 1D). Furthermore, we transplanted readily obtainable Lin− primitive hematopoietic cells from the bone marrow of 8- to 11-week-old CD45.2 mice into 8- to 11-week-old unconditioned congenic CD45.1 × CD45.2 mice. Fourteen weeks after the final transplantation of 6.5 × 108 purified Lin− cells over the course of 4 weekly injections, whereas the number of endogenous RUs was similar, the total number of RUs in bone marrow was approximately threefold higher than that in untransplanted animals (Figure 1E). In order to examine the progeny of the transplanted HSCs in unconditioned mice, we analyzed the donor chimerism in short-lived hematopoietic progenitors and mature blood cells. Flow cytometric analyses revealed that the donor chimerism within the GMP and CLP populations in bone marrow, as well as Gr-1hi granulocytes and B220hi B cells in peripheral blood, was similar to the donor HSC chimerism in unconditioned mice 13 weeks after the transplantation of 2.5 × 105 purified HSCs, indicating that transplanted donor HSCs generated blood cells normally in the bone marrow of unconditioned mice (Figure 1F). Furthermore, these results support the idea that cells in the CD34−CD48−LSK HSC population contribute to the large number of mature blood cells in bone marrow during homeostasis.8,9 Taken together, our results indicate that the number of empty HSC niches available for engraftment is larger than the number of niches occupied by endogenous HSCs, and also that most host HSCs were not replaced during the engraftment of donor HSCs into unconditioned mice upon transplantation.
Transplanted HSCs engraft into unconditioned bone marrow without HSC replacement. The chimerism in phenotypic HSCs (CD34−CD150+CD48−LSK) (A) and number of donor (CD45.2+) phenotypic HSCs (B) in the bone marrow of unconditioned CD45.1 × CD45.2 recipients 13 to 16 weeks after the transplantation of 2500 to 2.5 × 105 purified CD45.2 HSCs (n = 14). The numbers of donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total phenotypic HSCs (C) and functional HSCs (D-E) in untransplanted mice or unconditioned mice transplanted with 2.5 × 105 purified HSCs (C-D) (n = 3) or 6.5 × 108 purified Lin− cells (E) (n = 4). Functional HSCs were estimated using repopulating units (RUs) (D-E). The chimerism in phenotypic HSCs, GMPs, and CLPs in the bone marrow (BM), and Gr-1hi granulocytes, B220hi B cells, and CD3+ T cells in the peripheral blood (PB) of unconditioned recipients 13 weeks after the transplantation of 2.5 × 105 HSCs (F) (n = 3). All error bars represent standard deviations of the means. *P < .05.
Transplanted HSCs engraft into unconditioned bone marrow without HSC replacement. The chimerism in phenotypic HSCs (CD34−CD150+CD48−LSK) (A) and number of donor (CD45.2+) phenotypic HSCs (B) in the bone marrow of unconditioned CD45.1 × CD45.2 recipients 13 to 16 weeks after the transplantation of 2500 to 2.5 × 105 purified CD45.2 HSCs (n = 14). The numbers of donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total phenotypic HSCs (C) and functional HSCs (D-E) in untransplanted mice or unconditioned mice transplanted with 2.5 × 105 purified HSCs (C-D) (n = 3) or 6.5 × 108 purified Lin− cells (E) (n = 4). Functional HSCs were estimated using repopulating units (RUs) (D-E). The chimerism in phenotypic HSCs, GMPs, and CLPs in the bone marrow (BM), and Gr-1hi granulocytes, B220hi B cells, and CD3+ T cells in the peripheral blood (PB) of unconditioned recipients 13 weeks after the transplantation of 2.5 × 105 HSCs (F) (n = 3). All error bars represent standard deviations of the means. *P < .05.
Transplanted and endogenous HSCs show similar increases in expansion
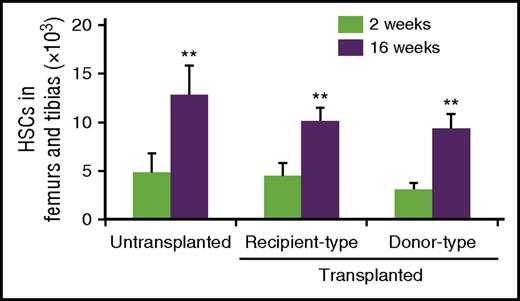
We next assessed the proliferation of HSCs that homed to empty HSC niches. A total of 1.6 × 108 purified Lin− cells, containing ∼2.4 × 105 CD34−CD48−LSK HSCs, from the bone marrow of 8-week-old CD45.2 mice, was intravenously injected into 8-week-old unconditioned congenic CD45.1 × CD45.2 mice, and the absolute numbers of HSCs were measured 2 and 16 weeks after transplantation. Flow cytometric analyses showed ∼8.1 × 103 donor CD34−CD48−LSK HSCs in femurs and tibias 2 weeks after transplantation, suggesting that ∼24% of HSCs injected into unconditioned recipients entered the bone marrow. Consistent with the previous studies,46-48 HSCs expanded with age (Figure 2). We found that donor and endogenous phenotypic HSCs (CD34−CD150+CD48−LSK) showed similar increases in expansion from 2 weeks to 16 weeks after transplantation in bone marrow, suggesting the presence of numerous empty proliferative sites for HSCs (Figure 2).
Transplanted and endogenous HSCs show similar increases in expansion. The numbers of donor (CD45.2+) and endogenous (CD45.1+CD45.2+) phenotypic HSCs (CD34−CD150+CD48−LSK) in the bone marrow of unconditioned CD45.1 × CD45.2 recipients 2 and 16 weeks after the transplantation of 1.6 × 108 purified CD45.2 Lin− cells or in the bone marrow of untransplanted mice (n = 4). All error bars represent standard deviations of the means. **P < .01.
Transplanted and endogenous HSCs show similar increases in expansion. The numbers of donor (CD45.2+) and endogenous (CD45.1+CD45.2+) phenotypic HSCs (CD34−CD150+CD48−LSK) in the bone marrow of unconditioned CD45.1 × CD45.2 recipients 2 and 16 weeks after the transplantation of 1.6 × 108 purified CD45.2 Lin− cells or in the bone marrow of untransplanted mice (n = 4). All error bars represent standard deviations of the means. **P < .01.
HSCs migrate from the bloodstream to sites distant from filled HSC niches
Our results showing that numerous empty HSC niches are available for engraftment and proliferation raised the question of whether empty HSC niches are adjacent to or distant from filled HSC niches. In order to address this question, we examined the location of HSCs in the bone marrow of unconditioned mice transplanted with a very large number of purified HSCs using a mouse strain that allowed for the ubiquitous, doxycycline-inducible expression of an H2B-GFP (in TetOP-H2B-GFP mice) to mark and visualize functional HSCs. Because HSCs are thought to divide infrequently, H2B-GFP label retention has been shown to correlate with long-term repopulation potential among hematopoietic stem and progenitor cells.43,49 Although long-term GFP-labeled cells were largely attributed to leaky background expression from a H2B-GFP strain,50,51 H2B-GFP+ cells are absent in untreated mice without doxycycline in the TetOP-H2B-GFP mice generated by Foudi et al.43 In TetOP-H2B-GFP mice, most functional HSCs were contained within the H2B-GFP+, but not the H2B-GFP− subpopulation of CD34−CD48−LSK cells 20 weeks after the administration of doxycycline (supplemental Figure 2A). Flow cytometric analyses revealed that H2B-GFP+ cells expressing c-kit (H2B-GFP+c-kit+ cells) strongly overlapped with the H2B-GFP+ subpopulation of CD34−CD48−LSK cells (supplemental Figure 2), and, thus, functional HSCs were defined as H2B-GFP+c-kit+ cells in the bone marrow of TetOP-H2B-GFP mice 20 weeks after the administration of doxycycline.
CD45.1 × CD45.2 TetOP-H2B-GFP mice were administered doxycycline and intravenously injected with 4.0 × 104 CD34−CD48−LSK HSCs stained with PKH26, which were sorted from the bone marrow of 8-week-old CD45.2 wild-type mice, 20 weeks after the administration of doxycycline (Figure 3A). Two days after transplantation, 1.6 × 103 PKH26+CD34−CD48−LSK cells were observed in femurs and tibias, suggesting that ∼29% of HSCs injected into unconditioned recipients entered the bone marrow. Immunohistochemical analyses of recipient bone marrow sections using antibodies against c-kit and GFP revealed that donor-derived PKH26+HSCs were scattered throughout the bone marrow cavity and occurred singly, but not in clusters, and also that single donor-derived PKH26+HSCs were located more than 40 μm away from other donor-derived PKH26+HSCs and endogenous H2B-GFP+c-kit+ cells (118 of 119; 99%) (Figure 3B and data not shown). Most donor-derived PKH26+HSCs (55 of 57; 97%) were in contact with CAR cells, defined as S100+ cells inside the marrow cavity42 (Figure 3C and data not shown).
Empty HSC niches available for engraftment are distant from filled HSC niches. Experimental design (A). Doxycycline-treated nonmyeloablated TetOP-H2B-GFP mice transplanted with 4.0 × 104 CD34−CD48−LSK cells stained with PKH26 (red) (B-C), were analyzed 20 weeks after the administration of doxycycline and 2 days after transplantation. Bone marrow sections from transplanted mice were stained with antibodies against GFP (green, in panel B), c-kit (dark blue, in panels B and C), and S100 (white, in panel C). Arrowheads indicate endogenous H2B-GFP+c-kit+ HSCs (B). Arrows indicate donor PKH26+ HSCs (B-C), which are in contact with S100+ CAR cells (C). The nuclei of cells were labeled with DAPI dye (white, in panel B). Experimental design (D). Doxycycline-treated nonmyeloablated TetOP-H2B-GFP mice transplanted with 1.6 × 108 TetOP-H2B-GFP mouse-derived purified Lin− cells were analyzed 20 weeks after the administration of doxycycline and 27 weeks after transplantation. The numbers of donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total H2B-GFP+c-kit+ HSCs (n = 4) (E). Half bones from transplanted mice were stained with antibodies against CD45.1 (red), GFP (green), and c-kit (dark blue), and representative images are shown (F). Arrows in panel F indicate donor CD45.1−H2B-GFP+c-kit+ HSCs, and arrowheads indicate endogenous CD45.1+H2B-GFP+c-kit+ HSCs. The nuclei of cells were labeled with DAPI dye (light blue). Half bones from transplanted mice were stained with antibodies against GFP (green) and c-kit (red) in panels G and H, S100 (white, in panel G), and CD31 (yellow, in panel H), and representative images are shown. Arrows indicate donor and endogenous H2B-GFP+c-kit+ HSCs (G-H). Arrowheads indicate CD31hi arterioles (H). Distances from donor and endogenous HSCs and random locations to the nearest CAR cells (I) or arteries (J). Using confocal microscopy, tiled Z-stacked optical sections (120-150 μm thick) were acquired from whole mount samples of half femurs (∼450-650 μm thick) (F-J). The statistical significance of differences between HSCs and random locations located within a 5-μm distance from CAR cells was determined by 2-tailed Student t tests (I). The statistical significance of overall differences in cell distribution was determined by Kolmogorov-Smirnov analysis (I-J). All error bars represent standard deviations of the means. **P < .01; ***P < .001.
Empty HSC niches available for engraftment are distant from filled HSC niches. Experimental design (A). Doxycycline-treated nonmyeloablated TetOP-H2B-GFP mice transplanted with 4.0 × 104 CD34−CD48−LSK cells stained with PKH26 (red) (B-C), were analyzed 20 weeks after the administration of doxycycline and 2 days after transplantation. Bone marrow sections from transplanted mice were stained with antibodies against GFP (green, in panel B), c-kit (dark blue, in panels B and C), and S100 (white, in panel C). Arrowheads indicate endogenous H2B-GFP+c-kit+ HSCs (B). Arrows indicate donor PKH26+ HSCs (B-C), which are in contact with S100+ CAR cells (C). The nuclei of cells were labeled with DAPI dye (white, in panel B). Experimental design (D). Doxycycline-treated nonmyeloablated TetOP-H2B-GFP mice transplanted with 1.6 × 108 TetOP-H2B-GFP mouse-derived purified Lin− cells were analyzed 20 weeks after the administration of doxycycline and 27 weeks after transplantation. The numbers of donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total H2B-GFP+c-kit+ HSCs (n = 4) (E). Half bones from transplanted mice were stained with antibodies against CD45.1 (red), GFP (green), and c-kit (dark blue), and representative images are shown (F). Arrows in panel F indicate donor CD45.1−H2B-GFP+c-kit+ HSCs, and arrowheads indicate endogenous CD45.1+H2B-GFP+c-kit+ HSCs. The nuclei of cells were labeled with DAPI dye (light blue). Half bones from transplanted mice were stained with antibodies against GFP (green) and c-kit (red) in panels G and H, S100 (white, in panel G), and CD31 (yellow, in panel H), and representative images are shown. Arrows indicate donor and endogenous H2B-GFP+c-kit+ HSCs (G-H). Arrowheads indicate CD31hi arterioles (H). Distances from donor and endogenous HSCs and random locations to the nearest CAR cells (I) or arteries (J). Using confocal microscopy, tiled Z-stacked optical sections (120-150 μm thick) were acquired from whole mount samples of half femurs (∼450-650 μm thick) (F-J). The statistical significance of differences between HSCs and random locations located within a 5-μm distance from CAR cells was determined by 2-tailed Student t tests (I). The statistical significance of overall differences in cell distribution was determined by Kolmogorov-Smirnov analysis (I-J). All error bars represent standard deviations of the means. **P < .01; ***P < .001.
Transplanted HSCs engraft into empty niches distant from niches filled by endogenous HSCs
In order to identify the location of homed HSCs long periods of time after the transplantation of HSCs into unconditioned mice, we injected 8-week-old unconditioned CD45.1 × CD45.2 TetOP-H2B-GFP mice intravenously with 1.6 × 108 purified Lin− cells from 8-week-old CD45.2 TetOP-H2B-GFP mice, administered doxycycline 1 week after transplantation, and analyzed the recipient bone marrow 27 weeks after transplantation (Figure 3D). Flow cytometric analyses revealed that, although the number of endogenous H2B-GFP+c-kit+ cells was similar, the total number of H2B-GFP+c-kit+ cells in recipient bone marrow was approximately twofold higher than that in untransplanted animals 20 weeks after the administration of doxycycline (Figure 3E). In order to image HSCs, we used a clearing method that enabled the deep imaging of bone marrow52 (supplemental Figure 3). Immunohistochemical analyses of recipient half bones revealed that H2B-GFP+c-kit+ cells (a ∼1:1 mixture of donor-derived and endogenous cells) were scattered throughout the bone marrow cavity and occurred singly but not in clusters, and also that single donor-derived CD45.1−H2B-GFP+c-kit+ cells were located more than 40 μm away from other donor-derived (CD45.1−) and endogenous (CD45.1+) H2B-GFP+c-kit+ cells 20 weeks after the administration of doxycycline (79 of 79; 100%) (Figure 3F and data not shown). Most donor-derived and endogenous H2B-GFP+c-kit+ cells (115 of 123; 93%) were in contact with CAR cells, defined as S100+ cells42 (Figure 3G and data not shown). In contrast, fewer H2B-GFP+c-kit+ cells were in contact with CD31hi morphologically identifiable arteries (0 of 121) (Figure 3H and data not shown). In transplanted mice as well as untransplanted mice, H2B-GFP+c-kit+ cells were significantly more likely to be close to CAR cells than in random locations (Figure 3I; supplemental Figure 4A). However, H2B-GFP+c-kit+ cells were slightly less likely to localize close to arteries than in random locations (Figure 3J; supplemental Figure 4B). This is consistent with the previous study by Acar et al,30 although it contradicts the study by Kunisaki et al.24 Similar results were obtained upon the observation of H2B-GFP+CD150+c-kit+ cells, which overlapped with the H2B-GFP+ subpopulation of CD34−CD150+LSK cells35,43,49 (data not shown). These results indicate that empty niches for HSCs that are available for engraftment are distant from niches occupied by endogenous HSCs.
HSC engraftment in nonmyeloablated bone marrow requires SCF produced by CAR cells
In order to determine the role of CAR cells in engraftment of HSCs in nonmyeloablated bone marrow, we transplanted CD34−CD48−LSK HSCs stained with PKH26 or readily obtainable Lin− primitive hematopoietic cells from the bone marrow of 8-week-old CD45.2 wild-type mice into 8-week-old unconditioned CD45.1 × CD45.2 control or Lepr-Cre;SCFf/f mice, in which SCF is depleted from CAR cells.38 Consistent with the prior study,38 the number of phenotypic HSCs (CD34−CD150+CD48−LSK) in bone marrow was reduced in 8-week-old Lepr-Cre;SCFf/f mice compared with control mice (data not shown). Two days after transplantation, the absolute number of donor-derived phenotypic HSCs in the bone marrow of Lepr-Cre;SCFf/f recipients was similar to that in control recipients (data not shown). Sixteen weeks after the transplantation of 1.6 × 108 purified Lin− cells into control or Lepr-Cre;SCFf/f mice, whereas the number of endogenous phenotypic HSCs was similar, the total number of phenotypic HSCs in bone marrow was approximately twofold higher than that in untransplanted animals (Figure 4). The numbers of donor-derived phenotypic HSCs as well as endogenous phenotypic HSCs were reduced in Lepr-Cre;SCFf/f recipients compared with control recipients (Figure 4), indicating that SCF produced by CAR cells is required chiefly for maintenance/expansion of HSCs in empty niches as well as niches for endogenous HSCs.
Engraftment of HSCs in unconditioned bone marrow, in which SCF is deleted from CAR cells. The numbers of donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total phenotypic HSCs (CD34−CD150+CD48−LSK) in untransplanted mice, and in control and Lepr-Cre;SCFf/f unconditioned recipients 16 weeks after the transplantation of 1.6 × 108 purified Lin− cells (n = 4). All error bars represent standard deviations of the means. *P < .05; **P < .01.
Engraftment of HSCs in unconditioned bone marrow, in which SCF is deleted from CAR cells. The numbers of donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total phenotypic HSCs (CD34−CD150+CD48−LSK) in untransplanted mice, and in control and Lepr-Cre;SCFf/f unconditioned recipients 16 weeks after the transplantation of 1.6 × 108 purified Lin− cells (n = 4). All error bars represent standard deviations of the means. *P < .05; **P < .01.
Endogenous myeloid progenitors are replaced by donor progenitors upon the transplantation of HSCs into nonmyeloablated mice
The total number of HSCs in the bone marrow was markedly increased by the transplantation of purified HSCs into unconditioned congenic mice; however, bone marrow cellularity remained unchanged from that in untransplanted animals (Figure 5A). These results prompted us to examine the absolute number of downstream multipotent and lineage-restricted progenitors in the bone marrow of 8- to 11-week-old unconditioned CD45.1 × CD45.2 mice transplanted with 2.5 × 105 purified HSCs from the bone marrow of 8- to 11-week-old CD45.2 mice, in which the total numbers of HSCs increased approximately twofold without replacing endogenous host HSCs by 13 weeks after transplantation. Flow cytometric analyses of the bone marrow revealed that, whereas the number of endogenous CD34+CD150+CD48−LSK short-term HSCs was similar, the total number of short-term HSCs in the bone marrow was approximately twofold higher than that in untransplanted animals (supplemental Figure 5). However, some endogenous CD34+CD150−CD48−LSK or CD34+CD48+LSK multipotent progenitors were replaced by donor multipotent progenitors derived from transplanted HSCs (supplemental Figure 5). Of note, flow cytometric analyses of GMPs revealed that, although the donor GMP chimerism was similar to the donor HSC chimerism (Figures 1F and 5B), the total number of GMPs, including donor and endogenous GMPs, was similar to that in untransplanted animals (Figure 5B). These results indicate that, in contrast to abundant empty HSC niches, presumptive GMP niches were saturated and endogenous GMPs were replaced by donor GMPs derived from transplanted HSCs.
Many endogenous myeloid progenitors are replaced by donor myeloid progenitors upon the transplantation of HSCs into unconditioned mice. Unconditioned CD45.1 × CD45.2 recipients were transplanted with 2.5 × 105 purified CD45.2 HSCs. Bone marrow was analyzed for the numbers of total nucleated cells (A) and donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total GMPs (B) 13 weeks after transplantation (n = 3). All error bars represent standard deviations of the means.
Many endogenous myeloid progenitors are replaced by donor myeloid progenitors upon the transplantation of HSCs into unconditioned mice. Unconditioned CD45.1 × CD45.2 recipients were transplanted with 2.5 × 105 purified CD45.2 HSCs. Bone marrow was analyzed for the numbers of total nucleated cells (A) and donor (CD45.2+), endogenous (CD45.1+CD45.2+), and total GMPs (B) 13 weeks after transplantation (n = 3). All error bars represent standard deviations of the means.
Discussion
Although it has been assumed that HSC niches are occupied by host endogenous HSCs, our results showed that upon the transplantation of purified HSCs into nonmyeloablated mice, many transplanted HSCs engrafted into recipient bone marrow, and the number of donor HSCs was larger than that of endogenous HSCs. The previous findings, obtained using a method similar to that of the present study, revealed that the number of donor HSCs residing in the bone marrow was ∼10-fold smaller than that of endogenous HSCs.14-16,19 This discrepancy may be explained by the fact that the absolute numbers of rare transplanted HSCs are difficult to measure in unconditioned recipient bone marrow transplanted with smaller numbers of purified HSCs. In addition, previous studies may potentially have encountered procedural issues, such as differences in the timeline of sorting, or damage to purified HSCs from donor mice for transplantation. Furthermore, the finding that myelosuppressive conditioning is required to achieve high levels of HSC engraftment11,13,15 has been explained, in part, by the idea that when extracellular immunological markers, including CD45, are used for transplantation studies, small immunological differences between the donor and host may necessitate myelosuppressive conditioning to prevent rejection.13,53
Our results indicate that there are numerous empty HSC niches available for the engraftment and proliferation of transplanted HSCs, which generate lympho-hematopoietic progenitors in bone marrow. The results from the histological analysis, revealing that donor and host HSCs occurred singly and that single HSCs were located in contact with CAR cells far from other HSCs, support the concept that all CAR (LepR+) cells create facultative HSC niches.1 This is consistent with the findings that the numbers of CAR (LepR1) cells expressing high levels of CXCL12 and SCF were markedly larger than HSC numbers,38,40,41 and that SCF produced by CAR cells is essential for HSC engraftment in niches distant from occupied HSC niches. This does not rule out the possibility that there is a unique niche for HSCs, because only 1 HSC interacted with 1 CAR cell, raising the possibility that an HSC may reside in a special site in the long processes and cell bodies of CAR cells. The appropriate localization of other types of cellular niches, including hematopoietic cells, may also be important for organizing niches for HSCs.32-34
It has been assumed that an increase in the number of stem cells is limited by the occupancy of a small group of their niches by endogenous stem cells, and that only when stem cells are lost or move away from their niches are they replaced by other stem cells.54 This study might refute these principles and raises a question about how the number of HSCs is regulated in abundant HSC niches. Consistent with our results, another recent study has shown that the massive reduction of HSCs does not induce expansion of residual HSCs.55 Together with the fact that HSCs increase with age,46-48 abundant HSC niches might support proliferation of mobile HSCs when their infrequent cell cycle entry is cell-autonomously induced. It will be important to determine how HSCs divide symmetrically to expand in facultative HSC niches created by CAR cells and endothelial cells during homeostasis.
We have shown that ∼26% of transplanted HSCs engrafted into unconditioned recipient bone marrow (Figure 1C), whereas ∼32% engrafted into irradiated bone marrow,30 suggesting that seeding efficiencies of intravenously transplanted HSCs are similar or slightly increased by irradiation.
In contrast to HSC engraftment, host GMPs were replaced by donor GMPs, which were generated from the transplanted HSCs, and overall GMP numbers remained unchanged upon the transplantation of purified HSCs into nonmyeloablated mice. These results suggest that GMP numbers are limited by the number of their saturable niches. However, GMP niches and the mechanisms involved in controlling the number of GMPs are still unclear and require further investigation.
Clinically, nonmyelosuppressive transplantation has many advantages because myelosuppressive conditioning causes extensive damage to other healthy tissues, as well as lifelong negative side effects, including secondary malignant diseases, ocular complications, lung diseases, and infertility, and is not desirable for many nonmalignant diseases. Although it has been assumed that myelosuppressive host conditioning is necessary to create space and open niches for transplanted HSCs, our results suggest that the availability of HSC niches does not have to be considered in order to improve the level of HSC engraftment in clinical marrow transplantation. However, injection of higher doses of HSCs, as compared with the current standard doses that are used in the myeloablative setting, is necessary to achieve equivalent levels of hematopoietic chimerism in unconditioned mice, because the number of endogenous HSCs remains unchanged in unconditioned recipients. An effective approach may be to develop methodologies that expand donor HSCs in vitro and in vivo,56,57 protect donor HSCs from damage during preparation,58 and enable immune rejection by recipients to be avoided. This study provides novel insights into the mechanisms underlying HSC maintenance and hematopoiesis and has important implications in clinical uses of HSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors appreciate the technical assistance provided by H. Watanabe, K. Kanari, and M. Yamaguchi, and thank Y. Yamada for secretarial assistance.
This work was supported by the Japan Science and Technology Agency and the Grant-in-Aid for Scientific Research (KAKENHI) of the Japanese Society for the Promotion of Science.
Authorship
Contribution: T.S. and T.N. conceived the project; M.S., T.S., and T.N. designed the experiments, analyzed the data, and wrote the manuscript; M.S. and T.S. performed the experiments; and T.N. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Nagasawa, Laboratory of Stem Cell Biology and Developmental Immunology, Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University, 1-3 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail: tnagasa@fbs.osaka-u.ac.jp.
References
Author notes
M.S. and T.S. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal