Whether maintenance therapy adds value in the treatment of HIV-associated multicentric Castleman disease (HIV+MCD) is unknown. In this issue of Blood, Dalla Pria et al have analyzed a prospective cohort to advocate that it is not.1
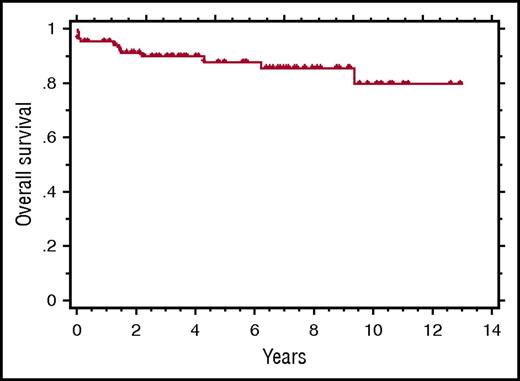
Kaplan-Meier curve showing the OS of 84 patients treated with rituximab-based immunotherapy for HIV+MCD. See Figure 2 in the article by Dalla Pria et al that begins on page 2143.
Kaplan-Meier curve showing the OS of 84 patients treated with rituximab-based immunotherapy for HIV+MCD. See Figure 2 in the article by Dalla Pria et al that begins on page 2143.
MCD is a potentially fatal systemic illness highly associated with HIV infection. In the setting of HIV, human herpesvirus 8 (HHV8) plays a critical role2 with the latent nuclear antigen-1 (LANA-1) expressed in the plasmablasts found within the B-cell follicles of pathologically enlarged nodes. Not a true malignancy, these plasmablasts are polyclonal, though monotypic for immunoglobulin M-κ.3,4 Despite the fact that the plasmablasts express only low levels of CD20,5 rituximab (the monoclonal antibody directed against CD20) induces complete remissions,6,7 likely by destroying the B cells elaborating interleukin-6 leading to the pathophysiology.8 With rituximab survival rates at 5 years improved from 33% to 90%.9
Despite this success, MCD is a relapsing illness in some patients. Because relapse can lead to death, some have advocated maintenance therapy.10 It is difficult to assess the efficacy of this strategy as the rate of relapse and the efficacy of rituximab reintroduction are unknown. Maintenance therapy is costly financially, inconvenient (IV), and associated with hypogammaglobulinemia in already immunocompromised patients.
To address this, Dalla Pria et al evaluated a prospective database of 84 HIV+MCD patients treated at Chelsea and Westminster Hospital (London, United Kingdom) since 2003. Notably, both initial and relapse disease were biopsy proven. This is important in a disease whose presentation overlaps with infection and lymphoma. Patients were treated in a risk-stratified approach with rituximab induction to which etoposide was added for poor performance status or defined end-organ damage including hemophagocytic syndrome or hemolytic anemia. Although 5% died within 2 weeks of therapy, underscoring the severity of MCD, 95% achieved a complete remission. Subsequently, 22% relapsed, but the median time to relapse was 30 months, ranging from 3 to 124 months. Moreover, relapses were successfully retreated with rituximab alone at this and subsequent relapses. In fact, all entered a second clinical remission and 9 have had a second relapse, 6 a third relapse, 4 a fourth relapse, and 3 a fifth relapse. Only 1 patient died of MCD.
One cannot dismiss the development of HHV8-associated lymphoma in 5 patients as it was fatal in 3. However, neither the lymphoma nor relapsed MCD was predictable by the parameters evaluated. Despite the risk of lymphoma, with a median follow-up of 6.9 years, the 5-year overall survival (OS) for the entire cohort was 88% (see figure). Presently, the benefit of maintenance therapy appears limited.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal