In this issue of Blood, Oliva et al report on a series of experiments that have demonstrated that in systemic light chain (AL) plasma cells (PCs), there is a complex interaction between those PCs and the immunoglobulin light chains (LCs) they produce.1 The pathology introduced by these unstable amyloidogenic LCs occurs not only at distant organs2,3 but also in the plasma cells that produce them. This manuscript attempts to unravel the relationship of AL LCs and the PCs that produce them in the context of AL PC susceptibility to proteasome inhibitors and the role autophagy may play in that susceptibility.
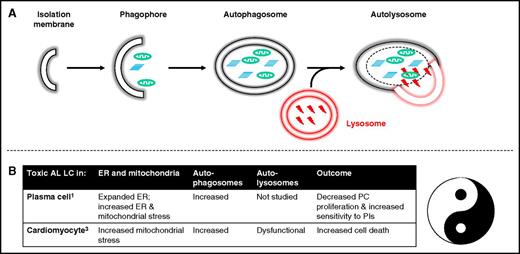
(A) Autophagy. An isolation membrane engulfs cytoplasm or organelle to form a double-membrane cytosolic vesicle referred to as an autophagosome. This autophagosome fuses with a late endosome or a lysosome to form an autophagolysosome. Inside the autophagolysosome, the lysosomal hydrolases degrade the sequestered material, which then becomes available to the cell for recycling. (B) Similar processes in plasma cells1 and cardiomyocytes3 have net opposing effects for the patient. Amyloidogenic immunoglobulin light chain induced dysregulated autophagy in a malignant PC favors cell death, which is good for the patient. In contrast, dysregulated autophagy in a cardiomyocyte also favors cell death, which is bad for the patient. ER, endoplasmic reticulum.
(A) Autophagy. An isolation membrane engulfs cytoplasm or organelle to form a double-membrane cytosolic vesicle referred to as an autophagosome. This autophagosome fuses with a late endosome or a lysosome to form an autophagolysosome. Inside the autophagolysosome, the lysosomal hydrolases degrade the sequestered material, which then becomes available to the cell for recycling. (B) Similar processes in plasma cells1 and cardiomyocytes3 have net opposing effects for the patient. Amyloidogenic immunoglobulin light chain induced dysregulated autophagy in a malignant PC favors cell death, which is good for the patient. In contrast, dysregulated autophagy in a cardiomyocyte also favors cell death, which is bad for the patient. ER, endoplasmic reticulum.
Macroautophagy, or autophagy, is a the coordinated catabolic process by which damaged and aged organelles and protein aggregates too large to be degraded by the ubiquitin-proteasome system are directed for lysosomal-mediated degradation (see figure panel A). Autophagy maintains organelle turnover and homeostasis. In addition to exerting control over cellular protein quality, autophagy is induced during nutritional deprivation, trophic factor withdrawal, and other types of cell stress to protect cells against apoptosis by degrading nonessential cell constituents for energy.4
The authors’ first observation is that AL PCs and multiple myeloma PCs both experience proteostatic stress as a result of their immunoglobulin production workload, but both types of PCs have similar proteasome capacity and workload.1 The higher sensitivity of AL PCs as compared with multiple myeloma PCs to a proteasome inhibitor appears to have less to do with their respective proteasome capacity (load vs capacity) than with their reduced autophagic control of organelle homeostasis. Using multiple myeloma PCs as controls, the authors demonstrate that in AL PCs, both the increased need for autophagy (as a result of endoplasmic reticulum stress and mitochondrial stress) and their autophagic impairment appear to be in part a result of the toxic AL LCs.
Mechanistically, these authors’ observations partially depart from other reports in the literature. The authors conclude that the abundant ER- and mitochondria-containing autophagosomes in AL PCs are a function of increased autophagy and/or of decreased lysosomal digestion of these autophagosomes, but favor the former as an explanation based on morphologic data.1 In contrast, in other models, data would point to diminished clearance/decreased lysosomal digestion as the major source of increased cellular autophagosomes.2,4 More work will be required in AL PCs to make this distinction.
Most important, the authors’ observations in AL PCs introduce a paradox for how one can translate their observations into treatment strategies for patients with AL amyloidosis. The same dysregulation of autophagy that makes AL PCs more sensitive to proteasome inhibition and contributes to impaired proliferation of AL PCs is a similar type of dysregulation of autophagy that others have demonstrated to be the source of toxicity in cardiomyocytes.2 For the AL PC, the goal would be to enhance (or perhaps merely to exploit) this impairment of autophagy; however, for organs the LCs target, such as the heart, the goal would be to enhance autophagy (see figure panel B). Clinical investigators are left with the question of whether autophagy activators such as rapamycin5 would be friend or foe: friend to the cardiomyocyte and the malignant plasma cell, but foe to the mission of eradicating the PC clone. Would one dare try an inhibitor of autophagy such as hydroxychloroquine to increase PC death for fear of decompensating a patient’s AL-involved heart further? Could some of the purported cardiac toxicity observed in some patients with AL amyloidosis treated with proteasome inhibitors be in part a result of this same mechanism?
Oliva and colleagues answer several interesting questions about the mechanisms of PI-induced AL PC death and the general processes occurring within these cells. Moreover, they offer an inducible in vitro murine PC model to further study these interactions of AL LCs in PCs and to potentially identify effective treatment strategies. This manuscript, taken in the context of the literature, however, is a potent reminder of the yin and yang of chemotherapy for patients with AL amyloidosis. It is well known that therapeutic indices are typically narrower in patients with AL amyloidosis because of their underlying organ dysfunction. What is not often considered is that agents that exploit the toxicity of AL LCs in bone marrow PC could potentially have a comparably negative effect on the cells of vulnerable organs the AL has already targeted.
Conflict-of-interest disclosure: A.D. has received clinical research dollars from Takeda, Pfizer, Alnylam, Celgene, and Prothena.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal